Tumour- and Non-Tumour-Associated Factors That Modulate Response to PD-1/PD-L1 Inhibitors in Non-Small Cell Lung Cancer
- PMID: 40647497
- PMCID: PMC12248828
- DOI: 10.3390/cancers17132199
Tumour- and Non-Tumour-Associated Factors That Modulate Response to PD-1/PD-L1 Inhibitors in Non-Small Cell Lung Cancer
Abstract
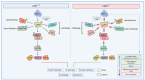
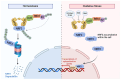
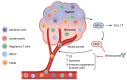
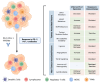
The interaction of programmed cell death receptor 1 (PD-1) on the surface of immune cells with its ligand, programmed cell death ligand 1 (PD-L1), expressed on tumour cells and antigen-presenting cells, leads to tumour immune evasion. Antibodies that target either PD-1 or its ligand PD-L1 have shown a favourable response in cancer patients, especially those with non-small cell lung cancer (NSCLC). However, only 15 to 25% of advanced NSCLC patients will benefit from immunotherapy. The PD-L1 tumour proportion score (TPS) is the current standard biomarker to select patients for PD-1/PD-L1 blockade therapy, as patients with a high PD-L1 TPS show better response compared to patients with a low PD-L1 TPS. However, since PD-L1 expression is a continuous variable and is an imperfect biomarker, investigation into additional predictive markers is warranted. This review focuses on tumour- and non-tumour-associated factors that have been shown to affect the response to PD-1/PD-L1 inhibitors in NSCLC. We also delve into mechanistic and clinical evidence on these potential biomarkers and their relationship to the tumour microenvironment (TME).
Keywords: NSCLC; PD-1; PD-L1; immune checkpoint inhibitors; patient response.
Conflict of interest statement
Dr. Tsao reports receiving grants from AZ and Sanofi; and personal fees from Daichii-Sankyo, AstraZeneca, Boehringer-Ingelheim, Abbvie, Sanofi, Pfizer and Diaceutics. M.K. declares no conflicts of interest.
Figures
Similar articles
-
Comprehensive Review: Unveiling the Pro-Oncogenic Roles of IL-1ß and PD-1/PD-L1 in NSCLC Development and Targeting Their Pathways for Clinical Management.Int J Mol Sci. 2023 Jul 17;24(14):11547. doi: 10.3390/ijms241411547. Int J Mol Sci. 2023. PMID: 37511306 Free PMC article.
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
Nivolumab for adults with Hodgkin's lymphoma (a rapid review using the software RobotReviewer).Cochrane Database Syst Rev. 2018 Jul 12;7(7):CD012556. doi: 10.1002/14651858.CD012556.pub2. Cochrane Database Syst Rev. 2018. PMID: 30001476 Free PMC article.
-
Comparison of efficacy and safety of PD-1/PD-L1 combination therapy in first-line treatment of advanced NSCLC: an updated systematic review and network meta-analysis.Clin Transl Oncol. 2024 Oct;26(10):2488-2502. doi: 10.1007/s12094-024-03442-3. Epub 2024 Apr 16. Clin Transl Oncol. 2024. PMID: 38625495
-
HMGB1 assists the predictive value of tumor PD-L1 expression for the efficacy of anti-PD-1/PD-L1 antibody in NSCLC.Cancer Chemother Pharmacol. 2025 Jan 24;95(1):28. doi: 10.1007/s00280-025-04751-2. Cancer Chemother Pharmacol. 2025. PMID: 39853458 Free PMC article.
Cited by
-
Use of PD-1/PD-L1 inhibitors in non-small cell lung cancer in elderly patients: a meta-analysis and systematic review of randomized clinical trials.Clin Transl Oncol. 2025 Aug 8. doi: 10.1007/s12094-025-04027-4. Online ahead of print. Clin Transl Oncol. 2025. PMID: 40779150 Review.
References
-
- Okazaki T., Maeda A., Nishimura H., Kurosaki T., Honjo T. PD-1 Immunoreceptor Inhibits B Cell Receptor-Mediated Signaling by Recruiting Src Homology 2-Domain-Containing Tyrosine Phosphatase 2 to Phosphotyrosine. Proc. Natl. Acad. Sci. USA. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. - DOI - PMC - PubMed
-
- Sheppard K.-A., Fitz L.J., Lee J.M., Benander C., George J.A., Wooters J., Qiu Y., Jussif J.M., Carter L.L., Wood C.R., et al. PD-1 Inhibits T-Cell Receptor Induced Phosphorylation of the ZAP70/CD3zeta Signalosome and Downstream Signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

