Current Landscape of Preclinical Models for Pediatric Gliomas: Clinical Implications and Future Directions
- PMID: 40647519
- PMCID: PMC12248539
- DOI: 10.3390/cancers17132221
Current Landscape of Preclinical Models for Pediatric Gliomas: Clinical Implications and Future Directions
Abstract
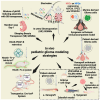
Pediatric high-grade gliomas (pHGGs), particularly diffuse midline gliomas (DMGs), are among the most lethal brain tumors due to poor survival and resistance to therapies. DMGs possess a distinct genetic profile, primarily driven by hallmark mutations such as H3K27M, ACVR1, and PDGFRA mutations/amplifications and TP53 inactivation, all of which contribute to tumor biology and therapeutic resistance. Developing physiologically relevant preclinical models that replicate both tumor biology and the tumor microenvironment (TME) is critical for advancing effective treatments. This review highlights recent progress in in vitro, ex vivo, and in vivo models, including patient-derived brain organoids, genetically engineered mouse models (GEMMs), and region-specific midline organoids incorporating SHH, BMP, and FGF2/8/19 signaling to model pontine gliomas. Key genetic alterations can now be introduced using lipofectamine-mediated transfection, PiggyBac plasmid systems, and CRISPR-Cas9, allowing the precise study of tumor initiation, progression, and therapy resistance. These models enable the investigation of TME interactions, including immune responses, neuronal infiltration, and therapeutic vulnerabilities. Future advancements involve developing immune-competent organoids, integrating vascularized networks, and applying multi-omics platforms like single-cell RNA sequencing and spatial transcriptomics to dissect tumor heterogeneity and lineage-specific vulnerabilities. These innovative approaches aim to enhance drug screening, identify new therapeutic targets, and accelerate personalized treatments for pediatric gliomas.
Keywords: GEMMs; diffuse midline gliomas; immunocompetent mouse model of DMGs; in utero electroporation; pHGGs.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

Similar articles
-
Combining the RCAS/tv-a retrovirus and CRISPR/Cas9 gene editing systems to generate primary mouse models of diffuse midline glioma.Neoplasia. 2025 Apr;62:101139. doi: 10.1016/j.neo.2025.101139. Epub 2025 Mar 7. Neoplasia. 2025. PMID: 40056601 Free PMC article.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Integrated analyses reveal two molecularly and clinically distinct subtypes of H3 K27M-mutant diffuse midline gliomas with prognostic significance.Acta Neuropathol. 2024 Sep 10;148(1):40. doi: 10.1007/s00401-024-02800-3. Acta Neuropathol. 2024. PMID: 39256213 Free PMC article.
-
Congress of Neurological Surgeons systematic review and evidence-based guidelines for the role of chemotherapy in newly diagnosed WHO Grade II diffuse glioma in adults: update.J Neurooncol. 2025 Jan;171(2):279-298. doi: 10.1007/s11060-024-04861-6. Epub 2024 Nov 20. J Neurooncol. 2025. PMID: 39565459
-
Pediatric Diffuse High-Grade Gliomas: A Comprehensive Review Of Ad-vanced Methods Of Diagnosis And Treatment.Curr Cancer Drug Targets. 2025 Jun 30. doi: 10.2174/0115680096365252250618115641. Online ahead of print. Curr Cancer Drug Targets. 2025. PMID: 40598730
References
-
- Ostrom Q.T., Price M., Ryan K., Edelson J., Neff C., Cioffi G., Waite K.A., Kruchko C., Barnholtz-Sloan J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2022;24:iii1–iii38. doi: 10.1093/neuonc/noac161. - DOI - PMC - PubMed
-
- Subramanian S., Ahmad T. StatPearls. StatPearls; Treasure Island, FL, USA: 2024. Childhood Brain Tumors. - PubMed
-
- Johnson K.J., Cullen J., Barnholtz-Sloan J.S., Ostrom Q.T., Langer C.E., Turner M.C., McKean-Cowdin R., Fisher J.L., Lupo P.J., Partap S., et al. Childhood brain tumor epidemiology: A brain tumor epidemiology consortium review. Cancer Epidemiol. Biomarkers Prev. 2014;23:2716–2736. doi: 10.1158/1055-9965.EPI-14-0207. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

