Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies
- PMID: 40647526
- PMCID: PMC12248532
- DOI: 10.3390/cancers17132228
Impact of Proton Pump Inhibitor Use on Progression-Free and Overall Survival in Cancer Patients Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis of Recent Studies
Abstract
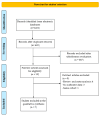
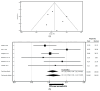
Background: The introduction of immunotherapy has significantly improved survival outcomes in many solid tumors. However, a subset of patients exhibits limited responsiveness to immune checkpoint inhibitors (ICIs). Emerging evidence indicates that the gut microbiota plays a critical role in modulating the effectiveness of immunotherapy. Consequently, the concurrent use of certain medications that disrupt microbial diversity may contribute to reduced treatment efficacy. Among the agents implicated in altering the gut microbiota are antibiotics and proton pump inhibitors (PPIs). Methods: A systematic literature search was conducted in PubMed, Scopus, and EMBASE. Eligible studies assessed the association between PPI use and progression-free survival (PFS) and/or overall survival (OS) in patients with solid tumors receiving ICIs. They reported hazard ratios (HRs) with 95% confidence intervals (CIs). The analysis focused on studies published between November 2022 and January 2025, in continuity with prior comprehensive meta-analyses that included studies up to November 2022. This contiguity-based approach enabled a focused evaluation of recent evidence, minimizing redundancy while allowing for the detection of evolving trends in clinical practice and methodology. Data were synthesized using both fixed-effects and random-effects models and visualized via Forest plots. Study quality was assessed using the Methodological Index for Non-Randomized Studies (MINORS) and the Newcastle-Ottawa Scale (NOS). Between-study heterogeneity and publication bias were evaluated using I2 statistics and funnel plots. Results: From a pool of over 400 screened articles between November 2022 and January 2025, seven studies met the inclusion criteria. The PFS analysis incorporated data from 1367 participants, while the OS analysis included 10,420 individuals. Use of PPIs was linked to a 12% higher risk of disease progression (HR = 1.12; 95% CI: 0.90-1.34) and an 18% increased mortality risk (HR = 1.18; 95% CI: 1.11-1.25). Conclusions: The observed association between PPIs exposure and reduced efficacy of ICIs, as reflected in worsened PFS and OS outcomes, highlights a potential clinical concern that merits further investigation in prospective studies.
Keywords: PPIs; immune checkpoint inhibitors; immunotherapy; microbiota; proton-pump inhibitors; solid tumors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
-
Proton pump inhibitors for the prevention of non-steroidal anti-inflammatory drug-induced ulcers and dyspepsia.Cochrane Database Syst Rev. 2025 May 8;5(5):CD014585. doi: 10.1002/14651858.CD014585.pub2. Cochrane Database Syst Rev. 2025. PMID: 40337979
-
Pharmacotherapy for anxiety and comorbid alcohol use disorders.Cochrane Database Syst Rev. 2015 Jan 20;1(1):CD007505. doi: 10.1002/14651858.CD007505.pub2. Cochrane Database Syst Rev. 2015. PMID: 25601826 Free PMC article.
-
Sequential versus standard triple first-line therapy for Helicobacter pylori eradication.Cochrane Database Syst Rev. 2016 Jun 28;2016(6):CD009034. doi: 10.1002/14651858.CD009034.pub2. Cochrane Database Syst Rev. 2016. PMID: 27351542 Free PMC article.
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article.
References
-
- Smith M., Dai A., Ghilardi G., Amelsberg K.V., Devlin S.M., Pajarillo R., Slingerland J.B., Beghi S., Herrera P.S., Giardina P., et al. Author Correction: Gut Microbiome Correlates of Response and Toxicity Following Anti-CD19 CAR T Cell Therapy. Nat. Med. 2023;29:2954. doi: 10.1038/s41591-022-02069-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

