Peroxisomal Alterations in Prostate Cancer: Metabolic Shifts and Clinical Relevance
- PMID: 40647540
- PMCID: PMC12248560
- DOI: 10.3390/cancers17132243
Peroxisomal Alterations in Prostate Cancer: Metabolic Shifts and Clinical Relevance
Abstract
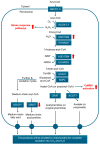
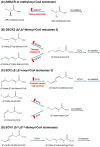
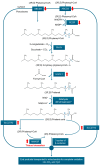
Cancer is hallmarked by uncontrolled cell proliferation and enhanced cell survival, driven by a complex interplay of factors-including genetic and epigenetic changes-that disrupt metabolic and signaling pathways and impair organelle function. While the roles of mitochondria and the endoplasmic reticulum in cancer are widely recognized, emerging research is now drawing attention to the involvement of peroxisomes in tumor biology. Peroxisomes are essential for lipid metabolism, including fatty acid α- and β-oxidation, the synthesis of docosahexaenoic acid, bile acids, and ether lipids, as well as maintaining redox balance. Despite their critical functions, the role of peroxisomes in oncogenesis remains inadequately explored. Prostate cancer (PCa), the second most common cancer in men worldwide, exhibits a unique metabolic profile compared to other solid tumors. In contrast to the glycolysis-driven Warburg effect, primary PCa relies primarily on lipogenesis and oxidative phosphorylation. Peroxisomes are intricately involved in the metabolic adaptations of PCa, influencing both disease progression and therapy resistance. Key alterations in peroxisomal activity in PCa include the increased oxidation of branched-chain fatty acids, upregulation of α-methylacyl coenzyme A racemase (a prominent PCa biomarker), and downregulation of 1-alkyl-glycerone-3-phosphate synthase and catalase. This review critically examines the role of peroxisomes in PCa metabolism, progression, and therapeutic response, exploring their potential as biomarkers and targets for therapy. We also consider their relationship with androgen receptor signaling. A deeper understanding of peroxisome biology in PCa could pave the way for new therapies to improve patient outcomes.
Keywords: androgen receptor; ether lipids; fatty acid oxidation; metabolic rewiring; peroxisomes; prostate cancer; redox homeostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.Eur Urol. 2014 Feb;65(2):467-79. doi: 10.1016/j.eururo.2013.11.002. Epub 2013 Nov 12. Eur Urol. 2014. PMID: 24321502
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

