Transarterial Chemoembolization Outperforms Radioembolization in Early- and Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Retrospective Study
- PMID: 40647551
- PMCID: PMC12249255
- DOI: 10.3390/cancers17132254
Transarterial Chemoembolization Outperforms Radioembolization in Early- and Intermediate-Stage Hepatocellular Carcinoma: A Multicenter Retrospective Study
Abstract
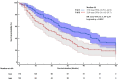
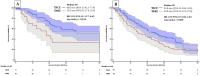
Background: Transarterial radioembolization (TARE) with Yttrium-90 microspheres is an established therapy for unresectable hepatocellular carcinoma (HCC). However, its clinical efficacy compared to transarterial chemoembolization (TACE) remains unclear. Methods: We retrospectively reviewed 279 consecutive patients undergoing TARE (n = 104) or TACE (n = 175) at four tertiary centers. Patients with metastatic disease, locally advanced HCC, or Child-Pugh (CP) C were excluded. Data on treatment, adverse events, survival outcomes (median overall survival [mOS], and objective response rates [by modified Response Evaluation Criteria in Solid Tumors; mRECIST]) were collected. Results: The median follow-up of the cohort was 27 months (IQR 13-50), the mean age was 67.6 ± 10.1 years, and 207 (74.2%) were male. The cohort was balanced in age, performance status, CP class, and HCC etiology. Maximum tumor diameter was significantly larger in the TARE cohort compared to the TACE cohort (4.4 vs. 3.1 cm, p < 0.001), including within the BCLC 0/A (4.2 vs. 2.7 cm, p = 0.001) and BCLC B (5.0 vs. 4.0 cm, p = 0.049) subgroups. The mOS was longer with TACE (37 vs. 22 months; hazard ratio [HR] 1.65, 95% CI: 1.19-2.29, p = 0.002). In BCLC 0/A patients, TACE yielded longer mOS (60 vs. 25 months; HR 2.35, 95% CI: 1.17-4.69; p = 0.016). In BCLC B, mOS was longer with TACE (32 vs. 20 months), but was not statistically significant (HR 1.39, 95% CI: 0.96-2.03, p = 0.080). In BCLC 0/A, complete response rates were higher with TACE (43.2% vs. 34.3%, p = 0.012). Hepatic decompensation was more frequent with TARE- (26.0%) than with TACE-treated patients (13.7%, p = 0.010). Conclusions: TACE demonstrated superior survival outcomes over TARE, particularly in early-stage disease. These results advocate for a more nuanced selection of embolization therapies in these patients.
Keywords: BCLC; Y90; decompensation; disease control; hepatocellular carcinoma; locoregional therapy; response; survival; transarterial chemoembolization; transarterial radioembolization; treatment.
Conflict of interest statement
Dr. Sanai consults for, and is on the speakers’ bureau of, Boston Scientific, Roche Pharmaceuticals, AstraZeneca, and Bayer-Schering. Others have nothing to disclose. Drs. Arabi and Khankan consult for, and are on the speakers’ bureau of Boston Scientific and Sirtex. Dr. Alzanbagi is on the speakers’ bureau of Boston Scientific and AstraZeneca.
Figures
References
-
- Alqahtani S.A., Sanai F.M., Alolayan A., Abaalkhail F., Alsuhaibani H., Hassanain M., Alhazzani W., Alsuhaibani A., Algarni A., Forner A., et al. Saudi Association for the Study of Liver diseases and Transplantation practice guidelines on the diagnosis and management of hepatocellular carcinoma. Saudi J. Gastroenterol. 2020;26((Suppl. 1)):S1–S40. doi: 10.4103/sjg.SJG_477_20. - DOI - PMC - PubMed
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Salem R., Lewandowski R.J., Mulcahy M.F., Riaz A., Ryu R.K., Ibrahim S., Atassi B., Baker T., Gates V., Miller F.H., et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: A comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous