Towards Precision Medicine in Sinonasal Tumors: Low-Dimensional Radiomic Signature Extraction from MRI
- PMID: 40647674
- PMCID: PMC12248528
- DOI: 10.3390/diagnostics15131675
Towards Precision Medicine in Sinonasal Tumors: Low-Dimensional Radiomic Signature Extraction from MRI
Abstract
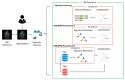
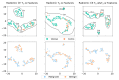
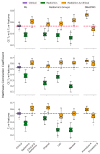
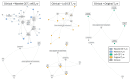
Background: Sinonasal tumors are rare, accounting for 3-5% of head and neck neoplasms. Machine learning (ML) and radiomics have shown promise in tumor classification, but current models lack detailed morphological and textural characterization. Methods: This study analyzed MRI data from 145 patients (76 malignant and 69 benign) across multiple centers. Radiomic features were extracted from T1-weighted (T1-w) images with contrast and T2-weighted (T2-w) images based on manually annotated tumor volumes. A dedicated ML pipeline assessed the effectiveness of different radiomic features and their integration with clinical variables. The DNetPRO algorithm was used to extract signatures combining radiomic and clinical data. Results: The results showed that ML classification using both data types achieved a median Matthews Correlation Coefficient (MCC) of 0.60 ± 0.07. The best-performing DNetPRO models reached an MCC of 0.73 (T1-w + T2-w) and 0.61 (T1-w only). Key clinical features included symptoms and tumor size, while radiomic features provided additional diagnostic insights, particularly regarding gray-level distribution in T2-w and texture complexity in T1-w images. Conclusions: Despite its potential, ML-based radiomics faces challenges in clinical adoption due to data variability and model diversity. Standardization and interpretability are crucial for reliability. The DNetPRO approach helps explain feature importance and relationships, reinforcing the clinical relevance of integrating radiomic and clinical data for sinonasal tumor classification.
Keywords: feature selection; machine learning; medical image analysis; otorhinolaryngology; radiomic.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Luong T.T., Yan C.H. Benign Paranasal Sinus Tumors. Curr. Otorhinolaryngol. Rep. 2023;11:332–343. doi: 10.1007/s40136-023-00466-1. - DOI
-
- Bracigliano A., Tatangelo F., Perri F., Di Lorenzo G., Tafuto R., Ottaiano A., Clemente O., Barretta M.L., Losito N.S., Santorsola M., et al. Malignant Sinonasal Tumors: Update on Histological and Clinical Management. Curr. Oncol. 2021;28:2420–2438. doi: 10.3390/curroncol28040222. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

