Blue Light and Green Light Fundus Autofluorescence, Complementary to Optical Coherence Tomography, in Age-Related Macular Degeneration Evaluation
- PMID: 40647687
- PMCID: PMC12248692
- DOI: 10.3390/diagnostics15131688
Blue Light and Green Light Fundus Autofluorescence, Complementary to Optical Coherence Tomography, in Age-Related Macular Degeneration Evaluation
Abstract
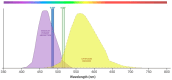
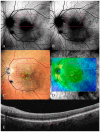
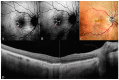
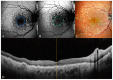
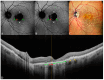
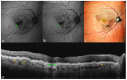
Background: Age-related macular degeneration (AMD) is one of the leading causes of permanent vision loss in the elderly, particularly in higher-income countries. Fundus autofluorescence (FAF) imaging is a widely used, non-invasive technique that complements structural imaging in the assessment of retinal pigment epithelium (RPE) integrity. While optical coherence tomography (OCT) remains the gold standard for retinal imaging due to its high-resolution cross-sectional visualization, FAF offers unique metabolic insights. Among the FAF modalities, blue light FAF (B-FAF) is more commonly employed, whereas green light FAF (G-FAF) provides subtly different image characteristics, particularly improved visualization and contrast in the central macula. Despite identical acquisition times and nearly indistinguishable workflows, G-FAF is notably underutilized in clinical practice. Objectives: This narrative review critically compares green and blue FAF in terms of their diagnostic utility relative to OCT, with a focus on lesion detectability, macular pigment interference, and clinical decision-making in retinal disorders. Methods: A comprehensive literature search was performed using the PubMed database for studies published prior to February 2025. The search utilized the keywords fundus autofluorescence and age-related macular degeneration. The primary focus was on short-wavelength FAF and its clinical utility in AMD, considering three aspects: diagnosis, follow-up, and prognosis. The OCT findings served as the reference standard for anatomical correlation and diagnostic accuracy. Results: Both FAF modalities correlated well with OCT in detecting RPE abnormalities. G-FAF demonstrated improved visibility of central lesions due to reduced masking by macular pigment and enhanced contrast in the macula. However, clinical preference remained skewed toward B-FAF, driven more by tradition and device default settings than by evidence-based superiority. G-FAF's diagnostic potential remains underrecognized despite its comparable practicality and subtle imaging advantages specifically for AMD patients. AMD stages were accurately characterized, and relevant images were used to highlight the significance of G-FAF and B-FAF in the examination of AMD patients. Conclusions: While OCT remains the gold standard, FAF provides complementary information that can guide management strategy. Since G-FAF is functionally equivalent in acquisition, it offers slight advantages. Broader awareness and more frequent integration of G-FAF that could optimize multimodal imaging strategies, particularly in the intermediate stage, should be developed.
Keywords: age-related macular degeneration; fundus autofluorescence; multimodal imaging; optical coherence tomography; retinal pigment epithelium; scanning laser ophthalmoscope.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
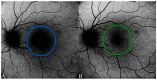
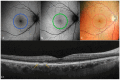
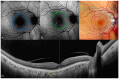
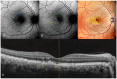

References
-
- Steinmetz J.D., Bourne R.R.A., Briant P.S., Flaxman S.R., Taylor H.R.B., Jonas J.B., Abdoli A.A., Abrha W.A., Abualhasan A., Abu-Gharbieh E.G., et al. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021;9:e144–e160. doi: 10.1016/S2214-109X(20)30489-7. - DOI - PMC - PubMed
-
- Wong W.L., Su X., Li X., Cheung C.M.G., Klein R., Cheng C.-Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Schmidt-Erfurth U., Chong V., Loewenstein A., Larsen M., Souied E., Schlingemann R., Eldem B., Monés J., Richard G., Bandello F. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA) Br. J. Ophthalmol. 2014;98:1144–1167. doi: 10.1136/bjophthalmol-2014-305702. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

