The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt
- PMID: 40647907
- PMCID: PMC12251675
- DOI: 10.3390/plants14131897
The Fusion Gene BPI-LY, Encoding Human Bactericidal/Permeability-Increasing Protein Core Fragments and Lysozyme, Enhanced the Resistance of Transgenic Tomato Plants to Bacterial Wilt
Abstract
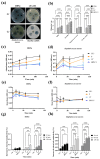
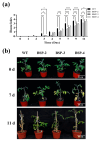
Tomato bacterial wilt, caused by Ralstonia solanacearum (G-), is one of the most devastating plant diseases. Developing effective resistance against this pathogen remains a major challenge in plant disease management. In this study, we constructed a fusion gene BPI-LY by combining the gene encoding the lipophilic functional domains of human bactericidal/permeability-increasing protein (BPI) with the gene of human lysozyme (LY). The recombinant gene BPI-LY was heterologously expressed in yeast and tomato. Preliminary in vitro assays in yeast demonstrated that BPI enhances LY's antibacterial activity against G- bacteria. Furthermore, overexpression of BPI-LY in tomato delayed onset of the disease in the transgenic lines and lowered the degree of tissue damage and the number of bacteria present in the stems relative to those in the wild-type plant. Additionally, the expression levels of the SlSOD, SlPOD, SlPAL, SlPR5, SlPR10, and SlPR-NP24 genes were indirectly upregulated in the transgenic plants following R. solanacearum inoculation. Collectively, these findings demonstrate that BPI-LY enhances the resistance of transgenic tomato against bacterial wilt caused by R. solanacearum.
Keywords: Ralstonia solanacearum; bacterial wilt; bactericidal/permeability increasing protein and lysozyme fusion gene (BPI-LY); disease resistance; tomato.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

