OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall
- PMID: 40647921
- PMCID: PMC12252322
- DOI: 10.3390/plants14131912
OsXTH19 Overexpression Improves Aluminum Tolerance via Xyloglucan Reduction in Rice Root Cell Wall
Abstract
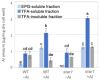
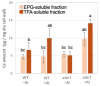
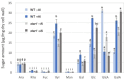
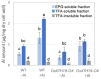
Aluminum (Al) dissolves from soil at low pH and is absorbed by plants, inhibiting their growth. Since most of the Al absorbed by plants is present in the cell wall, it is thought that the binding of Al to cell wall polysaccharides alters the properties of the cell wall and inhibits cell elongation. However, it remains unclear in which component of the cell wall Al accumulates. In this study, we determined the distribution of Al in rice root cell wall fractions under Al stress conditions. The results show that Al accumulates predominantly in the hemicellulose fraction, with star1 mutants accumulating significantly more Al than WT plants. An analysis of cell wall sugars revealed an increase in xyloglucan content under Al stress, which influenced the inhibition of root elongation. OsXTH19, a member of the xyloglucan endotransglucosylase/hydrolase (XTH) family, exhibits only xyloglucan endohydrolase (XEH) activity and lacks endotransglucosylase (XET) activity. OsXTH19 overexpressor rice (OsXTH19-OX) enhances the degradation of xyloglucan. Furthermore, OsXTH19-OX rice with reduced xyloglucan levels exhibited reduced Al accumulation and enhanced root growth under Al stress.
Keywords: Aluminum; Oryza sativa; OsXTH19; xyloglucan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Function of xyloglucan endotransglucosylase/hydrolases in rice.Ann Bot. 2014 Oct;114(6):1309-18. doi: 10.1093/aob/mct292. Epub 2013 Dec 19. Ann Bot. 2014. PMID: 24363334 Free PMC article.
-
XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis.Plant Cell. 2012 Nov;24(11):4731-47. doi: 10.1105/tpc.112.106039. Epub 2012 Nov 30. Plant Cell. 2012. PMID: 23204407 Free PMC article.
-
BnXTH1 regulates cadmium tolerance by modulating vacuolar compartmentalization and the cadmium binding capacity of cell walls in ramie (Boehmeria nivea).J Hazard Mater. 2024 May 15;470:134172. doi: 10.1016/j.jhazmat.2024.134172. Epub 2024 Mar 30. J Hazard Mater. 2024. PMID: 38569340
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Foy C.D. Plant Adaptation to Acid, Aluminum-toxic Soils. Commun. Soil Sci. Plant Anal. 1988;19:959–987. doi: 10.1080/00103628809367988. - DOI
-
- von Uexküll H.R., Mutert E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil. 1995;171:1–15. doi: 10.1007/BF00009558. - DOI
-
- Duan J., Gregory J. Coagulation by Hydrolysing Metal Salts. Adv. Colloid. Interface Sci. 2003;100:475–502. doi: 10.1016/S0001-8686(02)00067-2. - DOI
-
- Famoso A.N., Clark R.T., Shaff J.E., Craft E., McCouch S.R., Kochian L.V. Development of a Novel Aluminum Tolerance Phenotyping Platform Used for Comparisons of Cereal Aluminum Tolerance and Investigations into Rice Aluminum Tolerance Mechanisms. Plant Physiol. 2010;153:1678–1691. doi: 10.1104/pp.110.156794. - DOI - PMC - PubMed
-
- Kochian L.V. Cellular Mechanisms of Aluminum Toxicity and Resistance in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:237–260. doi: 10.1146/annurev.pp.46.060195.001321. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

