Phytochemical Evaluation and Antioxidant-Antimicrobial Potential of Lilium spp. Bulbs: Therapeutic and Dermatocosmetic Applications
- PMID: 40647926
- PMCID: PMC12251747
- DOI: 10.3390/plants14131917
Phytochemical Evaluation and Antioxidant-Antimicrobial Potential of Lilium spp. Bulbs: Therapeutic and Dermatocosmetic Applications
Abstract
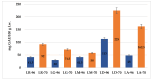
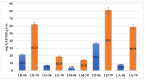
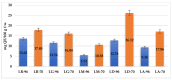
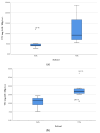
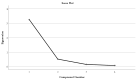
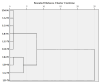
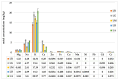
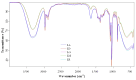
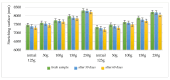
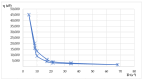
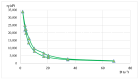
Lilium spp. bulbs are traditionally valued for their medicinal properties, yet their phytochemical profile and biomedical potential remain underexplored. This study aims to assess the antioxidant, antimicrobial, and dermatocosmetic potential of ethanolic macerates from five Lilium spp. cultivars. Bulb macerates were obtained using 70% and 96% ethanol and evaluated for total phenolic content (TPC), total flavonoid content (TFC), condensed tannins (CTC), mineral composition, and antioxidant activity (DPPH assay). Spectroscopic (FTIR) and antimicrobial analyses were also performed. Macerates from Lilium "Dark Secret" (LD-70) and Lilium asiaticum "White" (LA-70) exhibited the highest levels of TPC (225 and 162.5 mg GAE/100 g f.w.), TFC (26.12 and 21.75 mg QE/100 g f.w.), and antioxidant activity (81.5 and 58.75 mg GAE/100 g f.w.). FTIR confirmed the phenolic composition, while mineral analysis revealed a high potassium content and negligible toxic metals. Selective antimicrobial activity was observed against Escherichia coli, Pseudomonas aeruginosa, and Candida albicans, particularly for LD-70 and LA-70 macerates. Based on these findings, stable hydrogel formulations incorporating LD-70 and LA-70 were developed, showing favorable pH, rheology, and sustained antioxidant activity over 60 days. These findings support the integration of Lilium-derived macerates into dermatocosmetic formulations targeting skin protection and microbial defense.
Keywords: FTIR spectroscopy; Lilium spp.; antimicrobial activity; antioxidant activity; condensed tannins; flavonoids; maceration; phenolic compounds; phytocosmetics; skin health.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Unveiling the ethnomedicinal potential of Alchemilla speciosa Buser: An underexplored source of bioactive compounds for skin health.J Ethnopharmacol. 2025 Jul 24;351:120068. doi: 10.1016/j.jep.2025.120068. Epub 2025 May 30. J Ethnopharmacol. 2025. PMID: 40451493
-
Comprehensive phytochemical profiling of bioactive compounds from Barleria prattensis for their antioxidant and cytotoxic capacity and its characterization using GC-MS.Biochem Biophys Rep. 2025 Jun 10;43:102083. doi: 10.1016/j.bbrep.2025.102083. eCollection 2025 Sep. Biochem Biophys Rep. 2025. PMID: 40546346 Free PMC article.
-
Phytochemical profile and antimicrobial activity of individual frond extracts of Menisorus pauciflorus, Pteris catoptera, Conniogramme africana and Antrophyum mannianum.BMC Complement Med Ther. 2025 Jul 12;25(1):262. doi: 10.1186/s12906-025-05003-9. BMC Complement Med Ther. 2025. PMID: 40652226 Free PMC article.
-
A systematic review on natural products with antimicrobial potential against WHO's priority pathogens.Eur J Med Res. 2025 Jul 1;30(1):525. doi: 10.1186/s40001-025-02717-x. Eur J Med Res. 2025. PMID: 40597250 Free PMC article.
-
Selected honey as a multifaceted antimicrobial agent: review of compounds, mechanisms, and research challenges.Future Microbiol. 2025 May-Jun;20(7-9):589-610. doi: 10.1080/17460913.2025.2498233. Epub 2025 Apr 28. Future Microbiol. 2025. PMID: 40293032 Review.
References
-
- Liang Z.-X., Zhang J.-Z., Sun M.-Y., Zhang Y.-L., Zhang X.-H., Li H., Shi L. Variation of Phenolic Compounds and Antioxidant Capacities in Different Organs of Lilium pumilum. Nat. Prod. Commun. 2018;13:717–722. doi: 10.1177/1934578X1801300616. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

