An Experimental Approach for Investigating Freezing of Gait in Parkinson's Disease Using Virtual Reality and Neural Sensing: A Pilot Study
- PMID: 40648292
- PMCID: PMC12251975
- DOI: 10.3390/s25134036
An Experimental Approach for Investigating Freezing of Gait in Parkinson's Disease Using Virtual Reality and Neural Sensing: A Pilot Study
Abstract
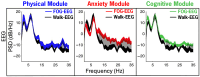
Freezing of gait (FOG) is a disabling symptom associated with Parkinson's disease (PD). Its understanding and effective treatment is compromised due to the difficulty in reliably triggering FOG in clinical and laboratory environments. The Cleveland Clinic-Virtual Home Environment (CC-VHE) platform was developed to address the challenges of eliciting FOG by combining an omnidirectional treadmill with immersive virtual reality (VR) environments to induce FOG under physical, emotional, and cognitive triggers. Recent developments in deep brain stimulation devices that sense neural signals from the subthalamic nucleus in real time offer the potential to understand the underlying neural mechanism(s) of FOG. This manuscript presents the coupling of the CC-VHE technology, VR paradigms, and the experimental and analytical methods for recording and analyzing synchronous cortical, subcortical, and kinematic data as an approach to begin to understand the nuanced neural pathology associated with FOG. To evaluate the utility and feasibility of coupling VR and neural sensing technology, initial data from one participant are included.
Keywords: Parkinson’s disease; deep brain stimulation; electroencephalography; freezing of gait; virtual reality.
Conflict of interest statement
The authors declare no related conflicts of interest.
Figures


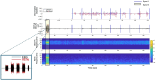
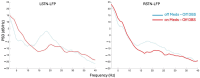
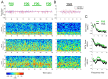
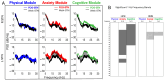
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

