An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement
- PMID: 40648786
- PMCID: PMC12249964
- DOI: 10.3390/jcm14134412
An Imaging-Based Marker to Refine Risk Stratification for Transcatheter Mitral Valve Replacement
Abstract
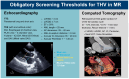
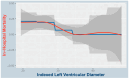
Background: The Tendyne™ transcatheter heart valve (THV) system is a promising option for high-risk patients with severe mitral regurgitation (MR) who are ineligible for surgery or transcatheter edge-to-edge repair (TEER). As most fatal complications occur within the first 90 days, this study aimed to identify anatomical predictors of in-hospital mortality after transcatheter mitral valve replacement (TMVR). Methods: In this subanalysis of the TENDER registry, data from 110 patients who underwent TMVR across 26 centers between January 2020 and June 2022 were evaluated. Preprocedural imaging parameters were analyzed, including transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and cardiac 4D computed tomography (CT). Results: We identified LVEDDi as a significant predictor of in-hospital mortality (p = 0.022), with lower values in non-survivors (26.42 ± 3.76 mm/m2) than in survivors (30.37 ± 5.58 mm/m2). Both indexed and absolute LVEDDi predicted in-hospital complications (p < 0.001 and p = 0.008). In multivariate analysis, LVEDDi (p = 0.048; OR = 0.856) and STS score (p = 0.038; OR = 1.114) remained independent predictors of in-hospital mortality. In an extended model, only LVEDDi persisted as a significant predictor (p = 0.007), highlighting its robustness. Conclusions: This analysis identified a small LVEDDi as a novel, clinically relevant risk factor in TMVR and showed its added value alongside conventional markers. Its easy calculation supports incorporating LVEDDi thresholds into screening to improve patient selection and outcomes.
Keywords: Tendyne™ valve system; left ventricular end-diastolic diameter index (LVEDDi); transcatheter mitral valve replacement (TMVR).
Conflict of interest statement
Elmar Kuhn declares conflicts of interest with Abbott (consulting, advisory, proctoring) and Medtronic (consulting, advisory, proctoring). Tillmann Kerbel received speaker honoraria from Abbott. Lenard Conradi reports advisory board membership and consulting activities for Abbott, Medtronic, JenaValve, Edwards Lifesciences, Boston Scientific, MicroPort, PiCardia, VenusMedtech, Neovasc, MicroInterventions, and Smartcanula Sarl. Andreas Zierer serves as a proctor for Abbott (Tendyne) and has received speaker honoraria and educational grants from Abbott. Francesco Maisano reports research and grant support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, Terumo, and VenusMedtech. He also reports personal consulting fees and honoraria from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Mtex, VenusMedtech, and Squadra. Additionally, he declares royalty income/IP rights from Edwards Lifesciences and equity ownership in Cardiogard, Cardiovalve, Magenta, SwissVortex, Transseptal Solutions, 4Tech, and Perifect. Marco Russo reports research support from Edwards Lifesciences. Andrea Colli is a proctor and consultant for Abbott. David Reineke reports travel support from Abbott, Edwards Lifesciences, and Medtronic and proctoring and consulting contracts with Abbott and Medtronic. Christophe Dubois reports consulting activities with Boston Scientific and Corcym and proctoring for Edwards Lifesciences. Jörg Hausleiter received speaker fees, research support, and advisory fees from Edwards Lifesciences. Ralph Stephan von Bardeleben serves as a speaker and advisor for Abbott Cardiovascular, Edwards Lifesciences, Medtronic, NeoChord, Philips, and Siemens. He also reports unpaid trial activity for Abbott Cardiovascular, Edwards Lifesciences, Jenscare, Medtronic, and NeoChord. Martin Andreas acts as a proctor, consultant, and speaker for Edwards, Abbott, Medtronic, Boston Scientific, Zoll, and Braun and has received institutional research grants from Edwards, Abbott, Medtronic, and LSI. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Muller D.W.M., Sorajja P., Duncan A., Bethea B., Dahle G., Grayburn P., Babaliaros V., Guerrero M., Thourani V.H., Bedogni F., et al. 2-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients With Severe Symptomatic Mitral Regurgitation. J. Am. Coll. Cardiol. 2021;78:1847–1859. doi: 10.1016/j.jacc.2021.08.060. - DOI - PubMed
-
- Guerrero M., Urena M., Himbert D., Wang D.D., Eleid M., Kodali S., George I., Chakravarty T., Mathur M., Holzhey D., et al. 1-Year Outcomes of Transcatheter Mitral Valve Replacement in Patients with Severe Mitral Annular Calcification. J. Am. Coll. Cardiol. 2018;71:1841–1853. doi: 10.1016/j.jacc.2018.02.054. - DOI - PubMed
-
- Duncan A., Daqa A., Yeh J., Davies S., Uebing A., Quarto C., Moat N. Transcatheter Mitral Valve Replacement: Long-Term Outcomes of First-in-Man Experience with an Apically Tethered Device- a Case Series from a Single Centre. EuroIntervention. 2017;13:e1047–e1057. doi: 10.4244/EIJ-D-17-00154. - DOI - PubMed
-
- Gössl M., Thourani V., Babaliaros V., Conradi L., Chehab B., Dumonteil N., Badhwar V., Rizik D., Sun B., Bae R., et al. Early Outcomes of Transcatheter Mitral Valve Replacement with the Tendyne System in Severe Mitral Annular Calcification. EuroIntervention. 2022;17:1523–1531. doi: 10.4244/EIJ-D-21-00745. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

