Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis
- PMID: 40648967
- PMCID: PMC12250057
- DOI: 10.3390/jcm14134590
Interleukin 23: Pathogenetic Involvement and Therapeutic Target for Ulcerative Colitis
Abstract
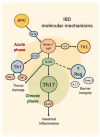
Interleukin-23 (IL-23) is a key cytokine involved in the pathogenesis of various immuno-mediated inflammatory diseases. In recent years, several drugs selectively targeting IL-23 have been developed and three of them (mirikizumab, risankizumab and guselkumab) were successfully investigated in clinical trials for ulcerative colitis (UC). All of them showed a good profile for efficacy, alleviating symptoms, and inducing endoscopic and histologic improvement, with very low incidence of adverse events. Bowel urgency also emerged as a crucial outcome from patients' perspective in the mirikizumab trials. The correct positioning of IL-23 inhibitors in the therapeutic algorithm for UC represents a new challenge for physicians, especially because it is not guided by biomarkers or predictors of effectiveness. Moreover, no comparative clinical data exist among the available IL-23 inhibitors, although molecular differences might potentially impact their effectiveness. A role for IL-23-inhibitors may also lie in combination with drugs with different mechanisms of action for complex, multi-refractory patients. This review, focusing on UC, summarizes all the clinical data available on IL-23 inhibitors and provides a perspective on the best clinical scenarios to maximize their effectiveness.
Keywords: Th17 lymphocytes; interleukin-23; monoclonal antibodies; ulcerative colitis.
Conflict of interest statement
Giuseppe Privitera received consultancy fees from Alphasigma and Janssen. Daniela Pugliese received speaker fees from MSD, Takeda, Galapagos, Janssen and Pfizer. No other relevant financial relationships or affiliations with any organization or entity with a financial interest in the subject matter discussed in this manuscript have been disclosed beyond those stated above.
Figures
References
-
- Macaluso F.S., Papi C., Orlando A., Festa S., Pugliese D., Bonovas S., Pansieri C., Piovani D., Fiorino G., Fantini M.C., et al. Use of Biologics for the Management of Crohn’s Disease: IG-IBD Clinical Guidelines Based on the GRADE Methodology. Dig. Liver Dis. 2023;55:442–453. doi: 10.1016/j.dld.2023.01.155. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources