Comparison of Two Initial Effect-Site Concentrations of Remifentanil with Propofol During Percutaneous Vertebroplasty Under Monitored Anesthesia Care: A Randomized Controlled Study with Titration-Based Adjustment
- PMID: 40649043
- PMCID: PMC12250791
- DOI: 10.3390/jcm14134669
Comparison of Two Initial Effect-Site Concentrations of Remifentanil with Propofol During Percutaneous Vertebroplasty Under Monitored Anesthesia Care: A Randomized Controlled Study with Titration-Based Adjustment
Abstract
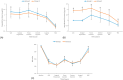
Background: Percutaneous vertebroplasty (PVP) is often performed under monitored anesthesia care (MAC) using a combination of propofol and remifentanil. However, the effects of different remifentanil effect-site concentrations (Ce) combined with propofol on perioperative outcomes in this procedure have not been reported. Methods: In this prospective, randomized controlled study, 80 patients scheduled for single-level PVP under MAC were enrolled. Participants were randomly assigned to receive propofol (Ce: 2.0 mcg/mL) combined with either a low (1.0 ng/mL; Group 1) or high (2.0 ng/mL; Group 2) remifentanil Ce. The primary outcome was the incidence of intraoperative patient movement; secondary outcomes included hemodynamic stability, perioperative adverse events, anesthetic consumption, frequency of dose adjustments, postoperative recovery, and anesthesia satisfaction. Results: Group 2 exhibited significantly fewer episodes of patient movement during the procedure and better intraoperative hemodynamic stability. Additionally, fewer upward adjustments in remifentanil infusion were observed in Group 2. Although the total propofol consumption was similar between the groups, Group 2 required a significantly lower propofol Ce to achieve adequate sedation. Surgeon satisfaction with anesthesia was also significantly higher in Group 2. Conclusions: Using a higher remifentanil Ce (2.0 ng/mL) in combination with propofol during PVP under MAC reduces patient movement and improves intraoperative hemodynamic stability without increasing adverse events. This regimen may thereby enhance procedural efficiency and surgeon satisfaction during vertebral interventions.
Keywords: monitored anesthesia care; percutaneous vertebroplasty; propofol; remifentanil; target-controlled infusion.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


References
-
- Klazen C.A., Lohle P.N., de Vries J., Jansen F.H., Tielbeek A.V., Blonk M.C., Venmans A., van Rooij W.J., Schoemaker M.C., Juttmann J.R., et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): An open-label randomised trial. Lancet. 2010;376:1085–1092. doi: 10.1016/S0140-6736(10)60954-3. - DOI - PubMed
LinkOut - more resources
Full Text Sources

