Comparison of Magnetic Resonance Imaging Scales for Assessment of Interval Changes of Arthropathy in Boys with Severe Hemophilia
- PMID: 40649172
- PMCID: PMC12251000
- DOI: 10.3390/jcm14134792
Comparison of Magnetic Resonance Imaging Scales for Assessment of Interval Changes of Arthropathy in Boys with Severe Hemophilia
Abstract
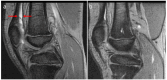
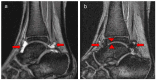
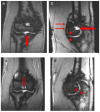
Background/Objectives: The variety of magnetic resonance imaging (MRI) scales available to measure soft tissue and osteochondral changes in joints of persons with hemophilia poses challenges in evaluating published clinical/research studies. To evaluate the value of four MRI scales [(i) the 17-point International Prophylaxis Study Group [IPSG] additive scale; (ii) and (iii) the compatible IPSG progressive (P) and additive (A) scales; and (iv) the Denver progressive scale] to assess joint change in boys with hemophilia participating in a prospective two-year prophylaxis study. Methods: Boys with severe hemophilia A (ages, 7-16 years) followed at the Hospital for Sick Children, Toronto, Canada had MRI evaluations of six index joints (ankles, knees, elbows) at study entry and exit. Musculoskeletal (MSK) outcomes included in the study were the Colorado Child Physical Examination (PE) scale; the Pettersson (X-ray) scale; and the aforementioned 4 MRI scales. Results: Very strong (r ≥ 0.80) correlations were observed between the IPSG 17-point, the IPSG progressive (P) and the Denver MRI scales, and moderate (r = 0.40-0.59) to strong (r = 0.60-0.79) correlations for the IPSG 17 point and the IPSG additive (A) MRI scales. Very weak (r = 0.20-0.39) or no correlations were observed between soft tissue MRI scores and the swelling item of the Child PE scale. Conclusions: All four MRI scales demonstrated relative comparability of their construct validities for assessing mild/moderate hemophilic arthropathy. The 17-point IPSG additive scale is recommended as a reference standard in future long-term studies of young boys with hemophilia receiving factor and non-factor-based preventive therapies.
Keywords: arthropathy; children; hemophilia; magnetic resonance imaging (MRI); prophylaxis.
Conflict of interest statement
Dr. Andrea Doria in the past three years has had the following relationships relevant to the disclosures of this manuscript: Advisory boards of the International Myositis Assessment & Clinical Studies Group (Chair, not for profit), OMERACT SIG in MRI in JIA and OMERACT Technical Advisory Group (Co-Chair, not for profit), and MIT Sloan Physicians’ Group (Co-Chair & Co-Founder, not for profit) and research support from The Terry Fox Foundation (Research Grant), NovoNordisk Health Care (Research Grant), the PSI Foundation (Research Grant), the Society of Pediatric Radiology (Research Grant), and the Garron Family Cancer Centre (Research Grant). Dr Victor Blanchette had received honoraria for participation in educational events and advisory boards sponsored by Sanofi-Genzyme and Takeda. He was a member of the Data Safety Monitoring Boards for Takeda. He received funding from Roche, Sanofi and Takeda for investigator-initiated studies. He was Chair of a non-profit International Prophylaxis Study Group that is funded by Educational grants from Bayer HealthCare, Biomarin, Novo Nordisk, Pfizer, Roche, Sanofi-Genzyme, Sobi and Takeda to the Hospital for Sick Children Foundation in Toronto, Canada. Dr. Blanchette passed away during the finalization stage of the manuscript review, but was the driving force for the completion of this work. Manuel Carcao reports receiving research support from Novartis, Novo Nordisk, Pfizer, Roche, Sanofi and Takeda and honoraria for speaking/participating in advisory boards from Bayer, LFB, Novo Nordisk, Pfizer, Roche, Sanofi and Takeda. He has no stocks or any equity in any pharmaceutical company and is not part of any company board. None of the authors had any financial interests in the conduct of this study profit.
Figures
Similar articles
-
Clotting factor concentrates for preventing bleeding and bleeding-related complications in previously treated individuals with haemophilia A or B.Cochrane Database Syst Rev. 2021 Aug 18;8(8):CD014201. doi: 10.1002/14651858.CD014201. Cochrane Database Syst Rev. 2021. PMID: 34407214 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Non-clotting factor therapies for preventing bleeds in people with congenital hemophilia A or B.Cochrane Database Syst Rev. 2024 Feb 27;2(2):CD014544. doi: 10.1002/14651858.CD014544.pub2. Cochrane Database Syst Rev. 2024. PMID: 38411279 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Exercise for haemophilia.Cochrane Database Syst Rev. 2016 Dec 19;12(12):CD011180. doi: 10.1002/14651858.CD011180.pub2. Cochrane Database Syst Rev. 2016. PMID: 27992070 Free PMC article.
References
-
- Fischer K., Poonnoose P., Dunn A.L., Babyn P., Manco-Johnson M.J., David J.A., van der Net J., Feldman B., Berger K., Carcao M., et al. Choosing outcome assessment tools in haemophilia care and research: A multidisciplinary perspective. Haemophilia. 2017;23:11–24. doi: 10.1111/hae.13088. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

