Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents
- PMID: 40649239
- PMCID: PMC12250624
- DOI: 10.3390/molecules30132720
Anti-Amyloid Aggregation Effects of Gobaishi (Galla chinensis) and Its Active Constituents
Abstract
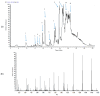
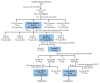
Alzheimer's disease (AD) is a chronic neurodegenerative disorder that leads to memory loss and changes in mental and behavioral functions in elderly individuals. A major pathological feature of AD is the aggregation of amyloid-beta (Aβ) peptides, along with oxidative stress, inducing neurocellular apoptosis in the brain. Gobaishi (Galla chinensis), a traditional herbal medicine, has gained considerable attention for its constituents and potent therapeutic properties, particularly its strong inhibitory activity against Aβ fibril formation. In this study, we investigated the anti-Aβ aggregation effects of Gobaishi and its active constituents. We isolated two compounds by employing Thioflavin T (ThT) assay-guided fractionation, which were identified through various spectroscopic methods as pentagalloyl glucose (PGG) and methyl gallate (MG). Evaluation of their anti-Aβ aggregation effects revealed that PGG and MG contribute 1.5% and 0.7% of the activity of Gobaishi, respectively. In addition, PGG demonstrated significantly stronger DPPH radical scavenging activity (EC50 = 1.16 µM) compared to MG (EC50 = 6.44 µM). At a concentration of 30 µM, PGG significantly reduced the Aβ-induced cytotoxicity in SH-SY5Y cell lines compared to MG. Based on these findings, both Gobaishi and its active compound PGG are proposed as promising candidates for further investigation as potent anti-amyloidogenic agents in AD management.
Keywords: Gobaishi; amyloid-beta; antioxidant; methyl gallate; pentagalloyl glucose.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Khoba K., Kumar S., Chatterjee S., Purty R.S. Isolation, characterization, and in silico interaction studies of bioactive compounds from Caesalpinia bonducella with target proteins involved in alzheimer′s disease. Appl. Biochem. Biotechnol. 2023;195:2216–2234. doi: 10.1007/s12010-022-03937-1. - DOI - PubMed
-
- World Health Organization Dementia. [(accessed on 17 October 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

