Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy
- PMID: 40649246
- PMCID: PMC12251241
- DOI: 10.3390/molecules30132727
Photophysical Properties and Protein Binding Studies of Piperazine-Substituted Anthracene-BODIPY Dyads for Antimicrobial Photodynamic Therapy
Abstract
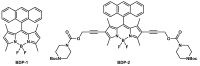
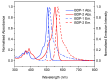
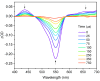
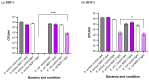
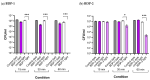
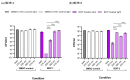
This work presents the synthesis, characterisation, photophysical properties, time-resolved spectroscopic behaviour, and biological evaluation of two structurally distinct heavy-atom-free BODIPY-anthracene dyads (BDP-1) and the newly designed 2,6-bis[1-(tert-butyl) 4-(prop-2-yn-1-yl) piperazine-1,4-dicarboxylate] BODIPY-anthracene (BDP-2), incorporating 2,6-alkynyl-piperazine substituents for potential application in antimicrobial photodynamic therapy. BDP-1 exhibits absorption and emission maxima at 507 nm and 516 nm, respectively, with a Stokes shift of 344 cm-1 in dichloromethane (DCM), characteristic of unsubstituted BODIPYs. In contrast, BDP-2 undergoes a red-shift in the absorption maximum to 552 nm (Stokes shift of 633 cm-1), which is attributed to the extended conjugation from the introduction of the alkyne groups. Time-resolved infrared spectroscopy confirmed efficient spin-orbit charge transfer intersystem crossing, and nanosecond transient absorption studies confirmed the formation of a long-lived triplet state for BDP-2 (up to 138 µs in MeCN). A binding constant (Kb) of 9.6 × 104 M-1 was obtained for BDP-2 when titrated with bovine serum albumin (BSA), which is higher than comparable BODIPY derivatives. BDP-2 displayed improved hemocompatibility compared to BDP-1 (<5% haemolysis of human erythrocytes up to 200 μg·mL-1). Antimicrobial activity of BDP-1 and BDP-2 was most potent when irradiated at 370 nm compared to the other wavelengths employed. However, BDP-2 did not retain the potent (6 log) and rapid (within 15 min) eradication of Staphylococcus aureus achieved by BDP-1 under irradiation at 370 nm. These findings demonstrate the rational design of BDP-2 as a biocompatible, and heavy-atom-free BODIPY offering promise for targeted antimicrobial photodynamic therapeutic applications.
Keywords: BODIPY; anthracene dyad; antimicrobial activity; fluorescence; heavy-atom-free; photodynamic therapy; triplet state.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
BODIPY-Loaded ZIF-8 Nanomaterials for Enhanced Photodynamic Inactivation of Staphylococcus aureus.ACS Appl Bio Mater. 2025 Jul 21;8(7):6316-6325. doi: 10.1021/acsabm.5c00787. Epub 2025 Jun 9. ACS Appl Bio Mater. 2025. PMID: 40488698
-
Enhancement of the triplet photosensitizing ability of BODIPY dyes by controlled red-shifted H-aggregate formation using ionic liquids: insights from spectroscopy, microscopy, DFT and biological studies.Phys Chem Chem Phys. 2025 Aug 20;27(33):17384-17398. doi: 10.1039/d5cp01890k. Phys Chem Chem Phys. 2025. PMID: 40757471
-
Facile synthesis and biological evaluation of [a]-benzannulated BODIPY derivatives as novel heavy-atom-free photosensitizers for photodynamic anticancer therapy.Bioorg Chem. 2025 Aug;163:108766. doi: 10.1016/j.bioorg.2025.108766. Epub 2025 Jul 18. Bioorg Chem. 2025. PMID: 40706538
-
Beclometasone for chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2013 Oct 9;2013(10):CD009769. doi: 10.1002/14651858.CD009769.pub2. Cochrane Database Syst Rev. 2013. PMID: 24105424 Free PMC article.
-
Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases.Cochrane Database Syst Rev. 2024 Jul 12;7(7):CD011778. doi: 10.1002/14651858.CD011778.pub2. Cochrane Database Syst Rev. 2024. PMID: 38994711 Free PMC article.
References
-
- WHO|No Time to Wait: Securing the Future from Drug-Resistant Infections. [(accessed on 21 October 2019)]; Available online: https://www.who.int/publications/i/item/no-time-to-wait-securing-the-fut....
-
- Home|AMR Review. [(accessed on 19 March 2020)]. Available online: https://amr-review.org/
-
- Tabrizi L., Hughes D.F., Pryce M.T. Covalent Organic Frameworks: Advancing Antimicrobial Photodynamic Therapy for next-Generation Treatments. Coord. Chem. Rev. 2025;528:216424. doi: 10.1016/j.ccr.2024.216424. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

