Recent Advances in the Mechanisms and Applications of Astragalus Polysaccharides in Liver Cancer Treatment: An Overview
- PMID: 40649307
- PMCID: PMC12250682
- DOI: 10.3390/molecules30132792
Recent Advances in the Mechanisms and Applications of Astragalus Polysaccharides in Liver Cancer Treatment: An Overview
Abstract
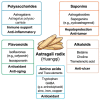
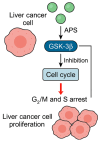
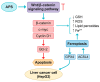
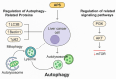
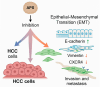
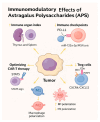
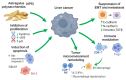
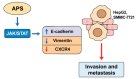
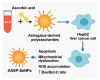
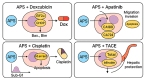
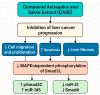
Astragalus polysaccharides (APS), bioactive compounds derived from Astragalus membranaceus, have emerged as promising natural agents in the treatment of hepatocellular carcinoma, a leading cause of cancer-related mortality. Preclinical studies indicate that APS exerts significant anti-liver cancer effects through multiple biological actions, including the promotion of apoptosis, inhibition of proliferation, suppression of epithelial-mesenchymal transition, regulation of autophagy, and modulation of immune responses. These therapeutic effects are closely associated with the regulation of critical signalling pathways, such as PI3K/AKT/mTOR, Wnt/β-catenin, JAK/STAT, and TGF-β/Smad. APS also reshapes the tumour microenvironment by enhancing macrophage activity, reducing the regulatory T cell function, and improving host immune response. In addition, APS exhibits synergistic effects when combined with conventional chemotherapeutics and interventional treatments such as transarterial chemoembolisation, improving efficacy and reducing toxicity. Despite the robust experimental evidence, limitations such as low bioavailability and a lack of large-scale clinical trials remain challenges for clinical translation. This review summarises the recent advances in understanding the anti-hepatocellular carcinoma activities of APS, their molecular targets and potential applications, aiming to provide a scientific basis for future studies and the development of APS-based therapeutic strategies.
Keywords: Astragalus polysaccharides; PI3K/AKT/mTOR; anti-liver cancer mechanisms; apoptosis; applications of Astragalus polysaccharides; immunomodulation; liver cancer; signalling pathways; traditional Chinese medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
- PL2024H002/Heilongjiang Province "double first-class" discipline collaborative innovation achievement project
- LJGXCG2023-089/Heilongjiang Province "double first-class" discipline collaborative innovation achievement project
- 2024-KYYWF-0617/Heilongjiang Province Education Department's Innovation Team
- JMSUGPZR2210/Jiamusi University's National Natural Science Foundation Cultivation Project
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

