Iron-Based High-Temperature Alloys: Alloying Strategies and New Opportunities
- PMID: 40649477
- PMCID: PMC12251445
- DOI: 10.3390/ma18132989
Iron-Based High-Temperature Alloys: Alloying Strategies and New Opportunities
Abstract
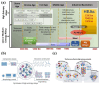
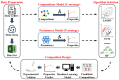
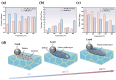
Iron-based high-temperature alloys are engineered to withstand extreme conditions, including elevated temperatures, mechanical stress, and corrosive environments. These alloys play a critical role in industries such as aerospace, power generation, and chemical processing, where materials must maintain structural integrity and performance under demanding operational conditions. This review examines recent advancements in iron-based alloys, with a focus on alloying strategies, high-temperature performance, and applications. Traditional approaches-including alloy design, microstructure control, process optimization, and computational modeling-continue to enhance alloy performance. Furthermore, emerging technologies such as high-entropy alloy (HEA) design, additive manufacturing (AM), nanostructured design with nanophase strengthening, and machine learning/artificial intelligence (ML/AI) are revolutionizing the development of iron-based superalloys, creating new opportunities for advanced material applications.
Keywords: Fe-based alloy; additive manufacturing; alloying strategy; high-entropy alloy; high-temperature application.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Stringer J., Wright I.G. Current Limitations of High-Temperature Alloys in Practical Applications. Oxid. Met. 1995;44:265–308. doi: 10.1007/BF01046730. - DOI
-
- Hart G.L.W., Mueller T., Toher C., Curtarolo S. Machine Learning for Alloys. Nat. Rev. Mater. 2021;6:730–755. doi: 10.1038/s41578-021-00340-w. - DOI
-
- Sharma P., Tucker W.C., Balasubramanian G. Optimal Interplay of Charge Localization, Lattice Dynamics and Slip Systems Drives Structural Softening in Dilute W Alloys with Re Additives. Int. J. Refract. Met. Hard Mater. 2025;128:107086. doi: 10.1016/j.ijrmhm.2025.107086. - DOI
-
- Meetham G.W. High-Temperature Materials—A General Review. J. Mater. Sci. 1991;26:853–860. doi: 10.1007/BF00576759. - DOI
-
- Eswarappa Prameela S., Pollock T.M., Raabe D., Meyers M.A., Aitkaliyeva A., Chintersingh K.-L., Cordero Z.C., Graham-Brady L. Materials for Extreme Environments. Nat. Rev. Mater. 2022;8:81–88. doi: 10.1038/s41578-022-00496-z. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

