Resolvin D2 and Its Effects on the Intestinal Mucosa of Crohn's Disease Patients: A Promising Immune Modulation Therapeutic Target
- PMID: 40649782
- PMCID: PMC12249623
- DOI: 10.3390/ijms26136003
Resolvin D2 and Its Effects on the Intestinal Mucosa of Crohn's Disease Patients: A Promising Immune Modulation Therapeutic Target
Abstract
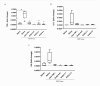
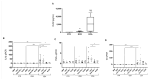
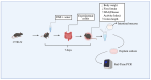
Crohn's disease (CD) is a chronic inflammatory disorder of the gastrointestinal tract that severely impacts patients' quality of life. Although current therapies have improved symptom management, they often fail to alter disease progression and are associated with immunosuppressive side effects. This study evaluated the immunomodulatory potential of resolvin D2 (RvD2), a pro-resolving lipid mediator, using a murine model of colitis and the ex vivo treatment of intestinal mucosal biopsies from CD patients, comparing its effects to those of conventional anti-TNFα therapy. To determine the optimal concentration of RvD2 for application in human tissue explant cultures, an initial in vitro assay was conducted using intestinal biopsies from mice with experimentally induced colitis. The explants were treated in vitro with varying concentrations of RvD2, and 0.1 μM emerged as an effective dose. This concentration significantly reduced the transcriptional levels of TNF-α (p = 0.004) and IL-6 (p = 0.026). Intestinal mucosal biopsies from fifteen patients with CD and seven control individuals were analyzed to validate RNA-sequencing data, which revealed dysregulation in the RvD2 biosynthetic and signaling pathways. The real-time PCR confirmed an increased expression of PLA2G7 (p = 0.02) and ALOX15 (p = 0.02), while the immunohistochemical analysis demonstrated the reduced expression of the RvD2 receptor GPR18 (p = 0.04) in intestinal tissues from CD patients. Subsequently, samples from eight patients with active Crohn's disease, eight patients in remission, and six healthy controls were used for the serum analysis of RvD2 by ELISA, in vitro treatment of intestinal biopsies with RvD2 or anti-TNF, followed by transcriptional analysis, and a multiplex assay of the explant culture supernatants. The serum analysis demonstrated elevated RvD2 levels in CD patients both with active disease (p = 0.02) and in remission (p = 0.002) compared to healthy controls. The ex vivo treatment of intestinal biopsies with RvD2 decreased IL1β (p = 0.04) and TNFα (p = 0.02) transcriptional levels, comparable to anti-TNFα therapy. Additionally, multiplex cytokine profiling confirmed a reduction in pro-inflammatory cytokines, including IL-6 (p = 0.01), IL-21 (p = 0.04), and IL-22 (p = 0.009), in the supernatant of samples treated with RvD2. Altogether, these findings suggest that RvD2 promotes the resolution of inflammation in CD and supports its potential as a promising therapeutic strategy.
Keywords: Crohn’s disease; immunoregulation; pro-resolving lipid mediator; resolvin D2.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet. 2018;390:2769–2778. doi: 10.1016/S0140-6736(17)32448-0. - DOI - PubMed
-
- Veauthier B., Hornecker J.R. Crohn’s disease: Diagnosis and management. Am. Fam. Phys. 2018;98:661–669. - PubMed
MeSH terms
Substances
Grants and funding
- 302557/2021-0 for R.F.L./National Council for Scientific and Technological Development (CNPq)
- 2022/09393-4 for R.F.L./São Paulo Research Foundation (FAPESP)
- #2332/20 for L.B.P./Funding for Education, Research and Extension Support (FAEPEX), University of Campinas
- Finance Code 001 for B.L.R./Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES Coor-denação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

