Type I Interferons in SARS-CoV-2 Cutaneous Infection: Is There a Role in Antiviral Defense?
- PMID: 40649826
- PMCID: PMC12249743
- DOI: 10.3390/ijms26136049
Type I Interferons in SARS-CoV-2 Cutaneous Infection: Is There a Role in Antiviral Defense?
Abstract
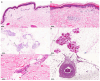
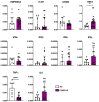
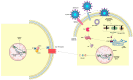
SARS-CoV-2, a β-coronavirus, primarily affects the lungs, with non-specific lesions and no cytopathic viral effect in the skin. Cutaneous antiviral mechanisms include activation of TLR/IRF pathways and production of type I IFN. We evaluated the antiviral mechanisms involved in the skin of COVID-19 patients, including skin samples from 35 deceased patients who had contracted COVID-19 before the launch of the vaccine. Detection of SARS-CoV-2 in the skin was performed using transmission electron microscopy and RT-qPCR. Microscopic and molecular effects of the virus in skin were evaluated by histopathology, RT-qPCR, and immunohistochemistry (IHC). The results revealed the presence of SARS-CoV-2 and microscopic changes, including microvascular hyaline thrombi, perivascular dermatitis, and eccrine gland necrosis. There was increased transcription of TBK1 and a reduction in transcription of TNFα by RT-qPCR in the COVID-19 group. IHC revealed reduced expression of ACE2, TLR7, and IL-6, and elevated expression of IFN-β by epidermal cells. In the dermis, there was decreased expression of STING, IFN-β, and TNF-α and increased expression of IL-6 in sweat glands. Our results highlight the role of type I IFN in the skin of COVID-19 patients, which may modulate the cutaneous response to SARS-CoV-2.
Keywords: COVID-19; SARS-CoV-2; STING; skin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

