Targets for CAR Therapy in Multiple Myeloma
- PMID: 40649828
- PMCID: PMC12249785
- DOI: 10.3390/ijms26136051
Targets for CAR Therapy in Multiple Myeloma
Abstract
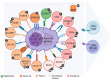
Multiple myeloma (MM or plasma cell myeloma) is a heterogenous B-cell malignant tumor that typically exhibits a high recurrence rate, resistance to drugs, and molecular diversity of tumor subclones. Given the limited efficacy of standard therapy options, cellular immunotherapy featuring a chimeric antigen receptor (CAR) has proven tangible potential in treatment for relapsed and refractory forms of MM. The rational choice of a tumor target which shows high selectivity, stable expression, and biological significance is key to the successful implementation of CAR therapy. This review has summarized and analyzed data from the literature on biological properties, the features of expression, and the clinical development stages of CAR cell products for MM treatment which target BCMA, GPRC5D, FcRH5, SLAMF7, CD38, CD138, TACI, APRIL, CD19, TNFR2, CD44v6, CD70, NKG2D ligands, etc. Special focus is on strategic approaches to overcoming antigenic escape, such as multi-specific CAR constructs, logical activation sequences, and controlled safety systems. The analysis underscores the need for integrating the molecular selection of targets with cutting-edge bioengineering solutions as a key trend for raising the efficacy, stability, and safety of cellular therapy in the case of MM.
Keywords: CAR-NK; CAR-T; antigen escape; cellular therapy; immunotherapy; logical activation; multi-specific CAR; multiple myeloma; safety of therapy; tumor targets.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

