Preclinical Evaluation of the Systemic Safety, Efficacy, and Biodistribution of a Recombinant AAV8 Vector Expressing FIX-TripleL in Hemophilia B Mice: Implications for Human Gene Therapy
- PMID: 40649847
- PMCID: PMC12250290
- DOI: 10.3390/ijms26136073
Preclinical Evaluation of the Systemic Safety, Efficacy, and Biodistribution of a Recombinant AAV8 Vector Expressing FIX-TripleL in Hemophilia B Mice: Implications for Human Gene Therapy
Abstract
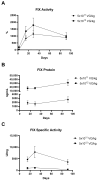
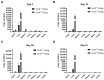
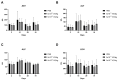
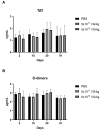
Gene therapy for hemophilia B offers the advantage of a single administration with sustained therapeutic effects. This study evaluated the systemic safety, efficacy, biodistribution, and immunogenicity of AAV8-FIX-TripleL, a recombinant adeno-associated virus type 8 (AAV8) vector encoding a modified factor IX (FIX) variant with increased activity. In this good laboratory practice (GLP)-compliant study, 180 male FIX-knockout hemophilia B mice were randomized into 12 groups (n = 15) and received intravenous AAV8-FIX-TripleL at therapeutic (5 × 1011 VG/kg) or supraphysiological (5 × 1012 VG/kg) doses on Day 1. The mice were sacrificed on Days 2, 15, 28, and 91 for comprehensive evaluations, including hematological and biochemical assessments, histopathological examination, FIX protein/activity analysis, immunogenicity assessment, and vector biodistribution via quantitative polymerase chain reaction (qPCR) in major organs. AAV8-FIX-TripleL demonstrated dose-dependent increases in FIX activity and protein levels, with FIX activity exceeding physiological levels and the maintenance of a favorable safety profile. Biodistribution analysis confirmed predominant hepatic accumulation and vector persistence up to 91 days post-injection, with minimal off-target distribution. These findings indicate that AAV8-FIX-TripleL is a promising gene therapy candidate for hemophilia B, as it has robust expression, sustained efficacy, and a favorable safety profile, and that further translational studies are warranted.
Keywords: AAV8; FIX; FIX-TripleL; gene therapy; hemophilia B.
Conflict of interest statement
Dr. Cheng-Po Huang, Ms. Ssu-Chia Wang, Ms. Yi-Hsiu Lin, Dr. Yen-Ting Chen, Ms. Jia-Yi Li, and Dr. Su-Yu Chen are employees of Trineo Biotechnology Co., Ltd., New Taipei City, Taiwan. All authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Manco-Johnson M.J., Abshire T.C., Shapiro A.D., Riske B., Hacker M.R., Kilcoyne R., Ingram J.D., Manco-Johnson M.L., Funk S., Jacobson L., et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N. Engl. J. Med. 2007;357:535–544. doi: 10.1056/NEJMoa067659. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

