Naturally Occurring Angiotensin Peptides Enhance the SARS-CoV-2 Spike Protein Binding to Its Receptors
- PMID: 40649848
- PMCID: PMC12249522
- DOI: 10.3390/ijms26136067
Naturally Occurring Angiotensin Peptides Enhance the SARS-CoV-2 Spike Protein Binding to Its Receptors
Abstract
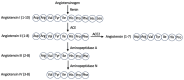
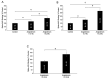
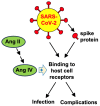
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), the virus responsible for Coronavirus Disease 2019 (COVID-19), utilizes its spike protein to infect host cells. In addition to angiotensin-converting enzyme 2 (ACE2) and neuropilin-1 (NRP1), AXL acts as a spike protein receptor and mediates infection, especially in respiratory cells with low ACE2 expression. Angiotensin II (1-8) can be cleaved into shorter peptides within the biological system. Antibody-based binding assays showed that angiotensin II causes a two-fold increase in the binding between the spike protein and AXL, but not ACE2 or NRP1. While a longer peptide, angiotensin I (1-10), did not affect the spike-AXL binding, shorter lengths of angiotensin peptides exhibited enhancing effects. The C-terminal deletions of angiotensin II to angiotensin (1-7) or angiotensin (1-6) resulted in peptides with enhanced activity toward spike-AXL binding with a similar capacity as angiotensin II. In contrast, the N-terminal deletions of angiotensin II to angiotensin III (2-8) or angiotensin IV (3-8) as well as the N-terminal deletions of angiotensin (1-7) to angiotensin (2-7) or angiotensin (5-7) produced peptides with a more potent ability to enhance spike-AXL binding (2.7-fold increase with angiotensin IV). When valine was substituted for tyrosine at position 4 in angiotensin II or when tyrosine at position 4 was phosphorylated, spike-AXL binding was increased, suggesting that modifications to tyrosine trigger enhancement. Angiotensin IV also enhances spike protein binding to ACE2 and NRP1. Thus, angiotensin peptides may contribute to COVID-19 pathogenesis by enhancing spike protein binding and thus serve as therapeutic targets.
Keywords: AXL; COVID-19; SARS-CoV-2; angiotensin; peptide; spike protein.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Update of
-
Angiotensin peptides enhance SARS-CoV-2 spike protein binding to its host cell receptors.bioRxiv [Preprint]. 2024 Dec 13:2024.12.12.628247. doi: 10.1101/2024.12.12.628247. bioRxiv. 2024. Update in: Int J Mol Sci. 2025 Jun 24;26(13):6067. doi: 10.3390/ijms26136067. PMID: 39713294 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

