Age-Associated Proteomic Changes in Human Spermatozoa
- PMID: 40649876
- PMCID: PMC12249680
- DOI: 10.3390/ijms26136099
Age-Associated Proteomic Changes in Human Spermatozoa
Abstract
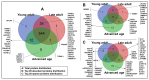
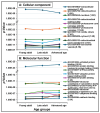
Advancing age in men significantly contributes to declining sperm fertility. Information on age-related proteomic changes in spermatozoa is limited. This study involved normal fertile Arab men in three age groups: young adult (21-30 years; n = 6), late adult (31-40 years; n = 7), and advanced age (40-51 years; n = 5). Gradient-purified spermatozoa were analyzed using LC-MS/MS and proteomic data were processed using Progenesis QI (QIfp) v3.0 and UniProt/SwissProt. Significantly enriched annotations and clustering of proteins in the proteomic datasets were identified (2-fold change; p < 0.05). A total of 588 proteins were identified, with 93% shared across the three groups. Unique proteins were MYLK4 for the young adult group, PRSS57 for the late adult group, and HMGB4, KRT4, LPGAT1, OXCT2, and MGRN1 for the advanced age group. Furthermore, 261 (44%) proteins were differentially expressed (p < 0.05) across the three groups. Functional enrichment analysis suggested an aging-related significant increase in pathways associated with neurodegenerative diseases and protein folding, alongside decreases in glycolysis/gluconeogenesis, flagellated sperm motility, acetylation, phosphoprotein modifications, oxidation processes, and Ubl conjugation. Cluster analysis highlighted significantly upregulated proteins in young adults (e.g., H2BC1, LAP3, SQLE, LTF, PDIA4, DYNLT2) and late adults (e.g., ATP5F1B, ODF2, TUBA3C, ENO1, SPO11, TEX45, TEKT3), whereas most proteins in the advanced age group exhibited downregulation (e.g., SPESP1, RAB10, SEPTIN4, RAB15, PTPN7, USP5, ANXA1, PRDX1). In conclusion, this study revealed aging-associated proteomic changes in spermatozoa that impact critical processes, including spermatogenesis, motility, metabolism, and fertilization, potentially contributing to fertility decline. These changes provide a molecular framework for developing therapies to preserve sperm proteostasis and enhance fertility in older men.
Keywords: LC-MS/MS; aging; human; proteomics; spermatozoa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Lahimer M., Montjean D., Cabry R., Capelle S., Lefranc E., Bach V., Ajina M., Ben Ali H., Khorsi-Cauet H., Benkhalifa M. Paternal age matters: Association with sperm criteria, spermatozoa DNA integrity and methylation profile. J. Clin. Med. 2023;12:4928. doi: 10.3390/jcm12154928. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

