Immunothrombosis in Sepsis: Cellular Crosstalk, Molecular Triggers, and Therapeutic Opportunities-A Review
- PMID: 40649892
- PMCID: PMC12249991
- DOI: 10.3390/ijms26136114
Immunothrombosis in Sepsis: Cellular Crosstalk, Molecular Triggers, and Therapeutic Opportunities-A Review
Abstract
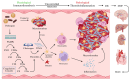
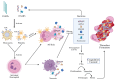
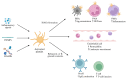
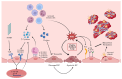
Sepsis remains a critical global health challenge characterized by life-threatening organ dysfunction arising from a dysregulated host response to infection. Immunothrombosis refers to the intersection of immune activation and coagulation pathways, particularly relevant in the context of sepsis. A growing body of evidence identifies immunothrombosis, a tightly interwoven process between innate immunity and coagulation. While immunothrombosis serves as a host defense mechanism under physiological conditions, its aberrant activation in sepsis precipitates microvascular thrombosis, organ ischemia, and progression toward disseminated intravascular coagulation (DIC). This review provides a comprehensive overview of the cellular contributors to immunothrombosis, including neutrophils, monocytes, platelets, and endothelial cells, and elucidates the signaling cascades, such as nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and inflammasome activation, that govern their interplay. We further highlight emerging molecular mediators, including extracellular traps, tissue factor expression, and cytokine amplification loops, that collectively promote pathological thromboinflammation. A deeper understanding of these interconnected pathways offers critical insights into the pathogenesis of sepsis and unveils potential targets for timely intervention. Ultimately, this review aims to bridge immunological and hematological perspectives to inform the development of novel therapeutic strategies against sepsis-induced coagulopathy.
Keywords: NETosis; cellular crosstalk; disseminated intravascular coagulation (DIC); immunothrombosis; sepsis; signaling cascades; therapeutic strategies.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

