Establishment of U-87MG Cellular Fibrosis as a Novel in Vitro Model to Analyze Glioblastoma Cells' Sensitivity to Temozolomide
- PMID: 40649899
- PMCID: PMC12249993
- DOI: 10.3390/ijms26136121
Establishment of U-87MG Cellular Fibrosis as a Novel in Vitro Model to Analyze Glioblastoma Cells' Sensitivity to Temozolomide
Abstract
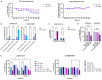
Glioblastoma (GBM), a highly malignant brain tumor, arises within a complex microenvironment that plays a critical role in facilitating tumor progression, ensuring survival, and enabling immune evasion, ultimately contributing to therapeutic resistance. Cancer-associated fibrosis is increasingly recognized as a key factor in the tumor pathophysiology, particularly in extracranial cancers, and reported therapeutic strategies in several cancers consist of the current use of the standard-of-care treatment combined with anti-fibrotic drugs. However, it remains unclear how the fibrotic changes associated with the GBM microenvironment contribute to the transformation of GBM from a chemosensitive state to a chemoresistant one. Here, we developed an in vitro model that mimics a fibrosis-like mechanism using the U-87MG GBM cell line. To achieve this, we identified the optimal experimental conditions (i.e., U-87MG cultured in serum-deprivation medium in the presence of recombinant TGF-B1 at 5 ng/mL for 72 h) that effectively induced fibrosis, as suggested by the counter-regulated expression of E- and N-cadherin and sustained levels of α-SMA and collagen I. As expected, U-87MG fibrotic cells were demonstrated to be more resistant to TMZ (predicted EC50 = 35 µM) as compared to the non-fibrotic counterpart (EC50 not achieved here; predicted EC50 = 351 µM). Accordingly, the anti-fibrotic uPAcyclin-a new derivative cyclic compound inspired as a A6 decapeptide drug-showed a significant cytotoxic effect, sensitizing resistant U-87MG fibrotic cells to TMZ. This highlights that targeting fibrosis may help to overcome TMZ resistance in GBM.
Keywords: GBM; chemoresistance; fibrosis; tumor-associated fibrotic alterations.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Marenco-Hillembrand L., Wijesekera O., Suarez-Meade P., Mampre D., Jackson C., Peterson J., Trifiletti D., Hammack J., Ortiz K., Lesser E., et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence-based analysis. J. Neurooncol. 2020;147:297–307. doi: 10.1007/s11060-020-03451-6. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

