Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity
- PMID: 40649975
- PMCID: PMC12249756
- DOI: 10.3390/ijms26136197
Neuroferritinopathy Human-Induced Pluripotent Stem Cell-Derived Astrocytes Reveal an Active Role of Free Intracellular Iron in Astrocyte Reactivity
Abstract
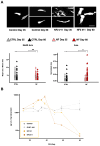
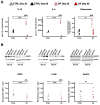
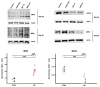
Increased iron levels, common in neurodegenerative diseases, correlate with disease severity, suggesting a role in the pathological process. Recently, efforts have been made to understand the role of iron in cerebral inflammatory processes. Employing astrocyte cell models of genetic neurodegenerative pathologies characterized by iron imbalance, such as the neurodegeneration with brain iron accumulation disorders, can provide valuable insights into astrocytes reactivity, a pivotal process in brain inflammation. Specifically, we employed human-induced pluripotent stem cell-derived astrocytes from Neuroferritinopathy, where iron accumulation is primary. After confirming iron accumulation and the deregulation of proteins involved in iron management, we observed that at 35 days since the beginning of differentiation, the elevated iron levels not only trigger ferroptosis but also place the astrocytes in a reactive state. This is evident in the higher extracellular concentrations of IL-6, IL-1β, and glutamate, along with changes in morphology, genes, and proteins involved in astrocyte reactivity. Interestingly, by day 60, IL-6 and IL-1β levels drop below those of the controls, and we observe a reversal in most of the factors considered. Moreover, at day 60, it is possible to observe not only increased senescence but also ferroptosis. These findings demonstrate that iron plays a primary role in inducing astrocyte reactivity.
Keywords: ferroptosis; iron homeostasis; neurodegenerative diseases; neuroferritinopathy; neuroinflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Curtis A.R.J., Fey C., Morris C.M., Bindoff L.A., Ince P.G., Chinnery P.F., Coulthard A., Jackson M.J., Jackson A.P., McHale D.P., et al. Mutation in the gene encoding ferritin light polypeptide causes dominant adult-onset basal ganglia disease. Nat. Genet. 2001;28:350–354. doi: 10.1038/ng571. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

