Immunomodulatory Mechanisms Underlying Neurological Manifestations in Long COVID: Implications for Immune-Mediated Neurodegeneration
- PMID: 40649991
- PMCID: PMC12249592
- DOI: 10.3390/ijms26136214
Immunomodulatory Mechanisms Underlying Neurological Manifestations in Long COVID: Implications for Immune-Mediated Neurodegeneration
Abstract
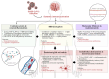
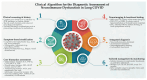
The COVID-19 pandemic has revealed the profound and lasting impact of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the nervous system. Beyond acute infection, SARS-CoV-2 acts as a potent immunomodulatory agent, disrupting immune homeostasis and contributing to persistent inflammation, autoimmunity, and neurodegeneration. Long COVID, or post-acute sequelae of SARS-CoV-2 infection (PASC), is characterized by a spectrum of neurological symptoms, including cognitive dysfunction, fatigue, neuropathy, and mood disturbances. These are linked to immune dysregulation involving cytokine imbalance, blood-brain barrier (BBB) disruption, glial activation, and T-cell exhaustion. Key biomarkers such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), glial fibrillary acidic protein (GFAP), and neurofilament light chain (NFL) correlate with disease severity and chronicity. This narrative review examines the immunopathological mechanisms underpinning the neurological sequelae of long COVID, focusing on neuroinflammation, endothelial dysfunction, and molecular mimicry. We also assess the role of viral variants in shaping neuroimmune outcomes and explore emerging diagnostic and therapeutic strategies, including biomarker-guided and immune-targeted interventions. By delineating how SARS-CoV-2 reshapes neuroimmune interactions, this review aims to support the development of precision-based diagnostics and targeted therapies for long COVID-related neurological dysfunction. Emerging approaches include immune-modulatory agents (e.g., anti-IL-6), neuroprotective drugs, and strategies for repurposing antiviral or anti-inflammatory compounds in neuro-COVID. Given the high prevalence of comorbidities, personalized therapies guided by biomarkers and patient-specific immune profiles may be essential. Advancements in vaccine technologies and targeted biologics may also hold promise for prevention and disease modification. Finally, continued interdisciplinary research is needed to clarify the complex virus-immune-brain axis in long COVID and inform effective clinical management.
Keywords: COVID-19; SARS-CoV-2; immune system; long COVID-19; neurological disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Post-acute sequelae SARS-CoV-2 infection and neuropathic pain: a narrative review of the literature and future directions.Pain Manag. 2025 Jun;15(6):333-343. doi: 10.1080/17581869.2025.2501521. Epub 2025 May 14. Pain Manag. 2025. PMID: 40366711 Review.
-
A multidimensional immunological perspective on long COVID.Cytokine Growth Factor Rev. 2025 Aug;84:1-11. doi: 10.1016/j.cytogfr.2025.07.001. Epub 2025 Jul 5. Cytokine Growth Factor Rev. 2025. PMID: 40640033 Review.
-
Long COVID and Biomarker Dysregulation-A Shift Toward Immune Exhaustion?Medicina (Kaunas). 2025 May 28;61(6):996. doi: 10.3390/medicina61060996. Medicina (Kaunas). 2025. PMID: 40572685 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19.Cochrane Database Syst Rev. 2021 Sep 2;9(9):CD013825. doi: 10.1002/14651858.CD013825.pub2. Cochrane Database Syst Rev. 2021. PMID: 34473343 Free PMC article.
Cited by
-
Potassium as an electro-metabolic signal for local coronary vasodilation.Basic Res Cardiol. 2025 Aug;120(4):815-833. doi: 10.1007/s00395-025-01126-9. Epub 2025 Jun 25. Basic Res Cardiol. 2025. PMID: 40560298
References
-
- Tsilingiris D., Vallianou N.G., Karampela I., Christodoulatos G.S., Papavasileiou G., Petropoulou D., Magkos F., Dalamaga M. Laboratory Findings and Biomarkers in Long COVID: What Do We Know So Far? Insights into Epidemiology, Pathogenesis, Therapeutic Perspectives and Challenges. Int. J. Mol. Sci. 2023;24:10458. doi: 10.3390/ijms241310458. - DOI - PMC - PubMed
-
- World Health Organization (WHO) A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. 2021. [(accessed on 1 April 2024)]. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_cond....
-
- Mudgal S.K., Gaur R., Rulaniya S., T L., Agarwal R., Kumar S., Varshney S., Sharma S., Bhattacharya S., Kalyani V. Pooled Prevalence of Long COVID-19 Symptoms at 12 Months and Above Follow-Up Period: A Systematic Review and Meta-Analysis. Cureus. 2023;15:e36325. doi: 10.7759/cureus.36325. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

