Clinical and Transcriptomic Characterization of Metastatic Hormone-Sensitive Prostate Cancer Patients with Low PTEN Expression
- PMID: 40650023
- PMCID: PMC12249740
- DOI: 10.3390/ijms26136244
Clinical and Transcriptomic Characterization of Metastatic Hormone-Sensitive Prostate Cancer Patients with Low PTEN Expression
Abstract
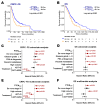
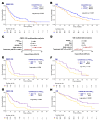
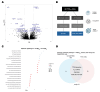
Alterations in the PTEN tumor suppressor gene are common in prostate cancer. They have been associated with a more aggressive disease and poor outcomes and potential benefit of targeted therapies. The purpose of this work is to study the clinical and transcriptional landscapes associated to low PTEN mRNA expression in metastatic hormone-sensitive prostate cancer (mHSPC) patients. A multicenter biomarker ambispective study was performed in mHSPC patients. PTEN mRNA expression was assessed by nCounter in formalin-fixed paraffin-embedded tumor samples. PTENlow status was defined by a previously validated cut-off and was correlated with castration-resistant prostate cancer (CRPC)-free survival (CRPC-FS) (primary endpoint) and overall survival (OS). RNA-Seq was performed to molecularly characterize PTENlow vs. PTENwt tumors. A total of 380 patients were included, 350 eligible. PTENlow was observed in 28.2% of patients and was independently associated with shorter CRPC-FS (HR 1.6, 95% CI 1.2-2.1, p = 0.002) and OS (HR 1.5, 95% CI 1.1-2, p = 0.014). PTENlow tumors showed overexpression of neuroendocrine, cell cycle, and DNA repair gene signatures, reduced expression of the androgen receptor pathway, and a distinct immune microenvironment. Using microarray data from the CHAARTED trial, we developed a PTEN-low related signature, independently associated with CRPC-FS (HR 1.5, 95% CI 1-2.3, p = 0.036) and OS (HR 1.9, C1 1.2-2.9, p = 0.005), and identified targets for potential therapies in PTEN-altered tumors. We conclude that PTENlow correlates with an aggressive clinical outcome in mHSPC patients and is associated with a unique transcriptional profile. These findings further support the investigation of novel therapeutic strategies for patients with PTEN alterations.
Keywords: CHAARTED trial; PTEN; androgen deprivation therapy; androgen receptor signaling inhibitors; biomarkers; docetaxel; hormone-sensitive prostate cancer.
Conflict of interest statement
The authors have provided the following conflicts to disclose (which may not be related to the subject matter of this manuscript): M.G.d.H.: speaker honoraria from Ipsen and travel and accommodation expenses from Novartis, Pfizer, and Bayer. C.A.: speaker honoraria from AstraZeneca, Pfizer, BMS, and Ipsen; travel accommodation from Pfizer, Ipsen, and Janssen. L.F.-M. (Laura Ferrer-Mileo): speaker honoraria from Pfizer, BMS, Ipsen, and Janssen; travel accommodation expenses from Pfizer and Janssen; research funding from Roche; advisory role from Pfizer. L.F.-M. (Laia Fernández-Mañas): speaker’s Bureau from Ipsen; financial relationships with commercial interests from Janssen, Ipsen, MSD, and Merck. A.F.: research funding from AstraZeneca; consulting or advisory role for Janssen, Astellas, and Bayer; travel and accommodation expenses from Janssen. A.R.-V.: research funding from Takeda, MSD, and Pfizer; consulting or advisory role for Astellas, Bayer, BMS, MSD, Janssen, Roche, Pfizer, and Clovis; honoraria or travel expenses from Pfizer, MSD, Astellas, BMS, Janssen, AstraZeneca, Roche, Bayer, and Sanofi Aventis. M.Á.C.: consulting or advisory role for BMS, MSD, Bayer, EUNSA, Pfizer, Roche, Janssen, Pierre Fabre, and Ipsen; travel and accommodation expenses from Janssen, Astellas, Roche, Ipsen, and MSD. S.C.: research funding from Pfizer and Janssen; consulting or advisory role for GSK; and speaker’s bureau for GSK, BMS, and Merck. I.C.: advisory boards for Pfizer, EISAI, and BMS. MF: speaker’s bureau for Pfizer, Ipsen, and Astellas; travel and accommodation expenses from Merck. NSG: advisory board for Pfizer, Bristol Myers Squibb, and Roche; speaker’s bureau for Ipsen and Astellas Pharma. A.P.: advisory and consulting fees from Roche, Pfizer, Novartis, Amgen, BMS, Puma, Oncolytics Biotech, MSD, Guardant Health, Peptomyc, and Lilly; lecture fees from Roche, Pfizer, Novartis, Amgen, BMS, Daiichi Sankyo, and Nanostring technologies; institutional financial interests from Boehringer, Novartis, Roche, Nanostring technologies, Sysmex Europe GmbH, Medica Scientia Innovation Research, SL, Celgene, Astellas, and Pfizer; leadership role in Reveal Genomics, SL; a patent PCT/EP2016/080056. Ò.R.: consulting or advisory role for BMS, EISAI, and Ipsen; travel and accommodation expenses from Ipsen and Pfizer. B.M.: research funding from Janssen, Roche, Bayer, and Pfizer; speaker’s bureau for Roche, Sanofi, Janssen, Astellas, Pfizer, Novartis, and Bristol-Myers Squibb; and travel and accommodation expenses from Janssen and Pfizer. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W.S., Parker C.C., Russell J.M., Attard G., et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

