Cloning, Expression and Functional Characterization of V. vinifera CAT2 Arginine Transporter
- PMID: 40650036
- PMCID: PMC12250071
- DOI: 10.3390/ijms26136259
Cloning, Expression and Functional Characterization of V. vinifera CAT2 Arginine Transporter
Abstract
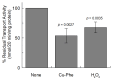
The amino acid membrane transporters of grape species take part in metabolic pathways that play crucial roles in nitrogen trafficking and in the synthesis of secondary metabolites. Therefore, identifying these amino acid transporters and defining their functional properties might have further applications in crop improvement and, hence, relevance to human nutrition. The VvCAT2 (Cation Amino acid Transporter) transporter cDNA has been isolated and cloned into a specific plasmid for over-expression in Escherichia coli. The expressed protein, after purification by Ni2+-chelating chromatography, has been functionally characterized in an experimental model of proteoliposomes by measuring the uptake of radiolabeled compounds. Arginine was revealed to be the best substrate, confirming the role of CAT2 in nitrogen trafficking in plant cells and within sub-cellular spaces, given its plausible localization in vacuoles. The transporter activity is modulated by pH, osmotic imbalance and ATP. The transport kinetics have been measured. Overall, the obtained data indicate the capacity of VvCAT2 in transporting arginine, making it a possible target for crop improvement with a relevance to human health.
Keywords: amino acid; plant nitrogen; secondary metabolites; transporter.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










References
-
- Brown K., Theofanous D., Britton R.G., Aburido G., Pepper C., Sri Undru S., Howells L. Resveratrol for the Management of Human Health: How Far Have We Come? A Systematic Review of Resveratrol Clinical Trials to Highlight Gaps and Opportunities. Int. J. Mol. Sci. 2024;25:747. doi: 10.3390/ijms25020747. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

