UBC9-Mediated SUMO Pathway Drives Prohibitin-1 Nuclear Accumulation and PITX1 Repression in Primary Osteoarthritis
- PMID: 40650059
- PMCID: PMC12249805
- DOI: 10.3390/ijms26136281
UBC9-Mediated SUMO Pathway Drives Prohibitin-1 Nuclear Accumulation and PITX1 Repression in Primary Osteoarthritis
Abstract
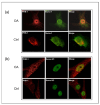
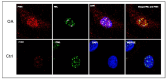
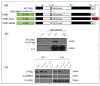
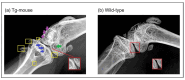
Osteoarthritis (OA) is a prevalent and debilitating joint disease in older adults with a complex etiology. We investigated the role of SUMOylation, a post-translational modification, in OA pathogenesis, focusing on the mitochondrial chaperone Prohibitin (PHB1) and the cartilage homeostasis transcription factor PITX1. We hypothesized that oxidative stress-induced SUMOylation promotes PHB1 nuclear accumulation, leading to PITX1 downregulation and contributing to OA development. Analysis of cartilage specimens from 27 OA patients and 4 healthy controls revealed an increased nuclear accumulation of PHB1 in OA chondrocytes, accompanied by elevated levels of SUMO-1 and SUMO-2/3. Mechanistically, nuclear PHB1 interacted indirectly with SUMO-1 through a SUMO-interacting motif (SIM), and the deletion of this SIM prevented PHB1 nuclear trapping in OA cells. Furthermore, the SUMO-conjugating enzyme E2 (UBC9) encoded by the UBE2I gene was upregulated in knee OA cartilage, and its overexpression in vitro enhanced PHB1 nuclear accumulation. Consistently, transgenic mice overexpressing the Ube2i gene exhibited increased UBC9 in their knee cartilage, resulting in Pitx1 downregulation and the emergence of an early OA-like phenotype in articular chondrocytes. Our findings uncover a novel role for UBC9-mediated SUMOylation in primary knee and hip OA. This pathway enhances PHB1 nuclear accumulation, contributing to PITX1 repression and subsequent OA development. These results underscore the importance of SUMOylation in OA pathogenesis and suggest potential molecular targets for early diagnosis and therapeutic intervention.
Keywords: PITX1; Prohibitin-1 (PHB1); SUMOylation; UBC9; osteoarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Tardio L., Andrés-Bergós J., Zachara N.E., Larrañaga-Vera A., Rodriguez-Villar C., Herrero-Beaumont G., Largo R. O-linked N-acetylglucosamine (O-GlcNAc) protein modification is increased in the cartilage of patients with knee osteoarthritis. Osteoarthr. Cartil. 2014;22:256–263. doi: 10.1016/j.joca.2013.12.001. - DOI - PubMed
-
- Catterall J.B., Hsueh M.F., Stabler T.V., McCudden C.R., Bolognesi M., Zura R., Jordan J.M., Renner J.B., Feng S., Kraus V.B. Protein modification by deamidation indicates variations in joint extracellular matrix turnover. J. Biol. Chem. 2012;287:4640–4651. doi: 10.1074/jbc.M111.249649. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

