Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved
- PMID: 40650204
- PMCID: PMC12250001
- DOI: 10.3390/ijms26136428
Oxidative Stress, MicroRNAs, and Long Non-Coding RNAs in Osteoarthritis Pathogenesis: Cross-Talk and Molecular Mechanisms Involved
Abstract
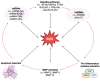
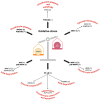
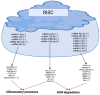
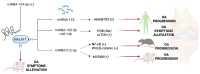
Osteoarthritis (OA) is the most common degenerative joint disease, characterized by articular cartilage degradation, synovial inflammation, and ligament lesions. Non-coding RNAs (ncRNAs) do not encode any protein products and play a fundamental role in regulating gene expression in several physiological processes, such as in the regulation of cartilage homeostasis. When deregulated, they affect the expression of genes involved in cartilage degradation and synovial inflammation, contributing to the onset and progression of OA. Oxidative stress is also involved in the pathogenesis of OA by contributing to the inflammatory response, degradation of the extracellular matrix, and induction of chondrocyte apoptosis. Studies in the literature show a reciprocal relationship between the altered expression of a number of ncRNAs, including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), and oxidative stress. The aim of this review is to highlight the role of oxidative stress, miRNAs, and lncRNAs and their cross-talk in OA in order to understand the main molecular mechanisms involved and to identify possible targets that may be useful for the identification and development of new diagnostic and therapeutic approaches for this disease.
Keywords: cartilage degradation; lncRNAs; miRNAs; osteoarthritis; oxidative stress.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
MicroRNAs play a role in chondrogenesis and osteoarthritis (review).Int J Mol Med. 2014 Jul;34(1):13-23. doi: 10.3892/ijmm.2014.1743. Epub 2014 Apr 15. Int J Mol Med. 2014. PMID: 24736803
-
Role and mechanism of lncRNA ZEB1-AS1 endogenous competition for miR-365a-3p targeting NRF2 in ferroptosis in articular chondrocytes.J Mol Histol. 2025 Jun 7;56(3):190. doi: 10.1007/s10735-025-10450-2. J Mol Histol. 2025. PMID: 40481906
-
Identification of Genes Linked to Meniscal Degeneration in Osteoarthritis: An In Silico Analysis.Int J Mol Sci. 2025 Jul 11;26(14):6651. doi: 10.3390/ijms26146651. Int J Mol Sci. 2025. PMID: 40724902 Free PMC article.
-
Long Non-coding RNA RASSF8-AS1 Promotes M1 Macrophage Polarization in Osteoarthritis via Moderating miR-27a-3p.Tohoku J Exp Med. 2025 Jul 9;266(3):219-227. doi: 10.1620/tjem.2024.J092. Epub 2024 Sep 12. Tohoku J Exp Med. 2025. PMID: 39261080
-
Multidimensional communication of microRNAs and long non-coding RNAs in lung cancer.J Cancer Res Clin Oncol. 2019 Jan;145(1):31-48. doi: 10.1007/s00432-018-2767-5. Epub 2018 Nov 11. J Cancer Res Clin Oncol. 2019. PMID: 30417217 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

