Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer
- PMID: 40650229
- PMCID: PMC12250494
- DOI: 10.3390/ijms26136452
Emerging Therapeutic Strategies Targeting GPX4-Mediated Ferroptosis in Head and Neck Cancer
Abstract
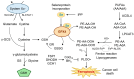
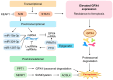
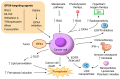
Ferroptosis, a regulated form of iron-dependent lipid peroxidation-induced cell death, has emerged as a compelling therapeutic strategy to overcome treatment resistance in head and neck cancer (HNC). Glutathione peroxidase 4 (GPX4), a selenoenzyme responsible for detoxifying phospholipid hydroperoxides, plays a central role in blocking ferroptosis and is frequently upregulated in therapy-resistant HNC subtypes. In this review, we examine the multifaceted regulation of GPX4 expression and function, including transcriptional, post-transcriptional, epigenetic, and proteostatic mechanisms. We explore how GPX4 suppression through pharmacologic inhibitors (e.g., RSL3, withaferin A, statins), metabolic stress, or combined therapies (e.g., radiotherapy, EGFR inhibitors, immunotherapy) induces ferroptosis and resensitizes resistant tumors. We also summarize emerging biomarkers, including GPX4, ACSL4, SLC7A11, and NCOA4, that predict ferroptosis sensitivity and may guide patient selection for ferroptosis-targeted therapies. Single-cell and spatial transcriptomics reveal significant intratumoral heterogeneity in ferroptosis susceptibility, underscoring the need for precision approaches. Despite promising preclinical data, challenges such as drug delivery, toxicity, and resistance mechanisms remain. Nevertheless, the ferroptosis-GPX4 axis represents a unique vulnerability in HNC that can be therapeutically exploited. Integrating ferroptosis modulation into personalized oncology may transform outcomes for patients with refractory disease.
Keywords: GPX4; ferroptosis; head and neck cancer; lipid peroxidation; therapy resistance.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

