A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis
- PMID: 40650247
- PMCID: PMC12250416
- DOI: 10.3390/ijms26136472
A Critical Role of Neutrophil-Driven Amplification of Chronic Microinflammation in the Biocompatibility of Hemodialysis
Abstract
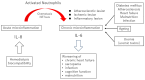
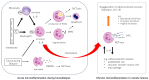
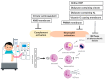
This review highlights recent insights into the pathophysiology and therapeutic strategies for improving biocompatibility in hemodialysis. Hemodialysis activates the innate immune system, particularly the complement cascade and neutrophils, leading to acute microinflammation. Interleukin-8 (IL-8), which increases during dialysis, promotes neutrophil chemotaxis and neutrophil extracellular trap (NET) formation, triggering myeloperoxidase (MPO) release and oxidative stress. Neutrophil accumulation in atherosclerotic plaques exacerbates vascular inflammation through IL-6 upregulation. Elevated levels of IL-8, MPO, and NET-related biomarkers are associated with increased all-cause and cardiovascular mortality in dialysis patients. Strategies to mitigate these effects include the use of advanced membrane materials (e.g., AN69, vitamin E-coated, polymethyl methacrylate), novel dialysis modalities (e.g., high-volume online hemodiafiltration, cool dialysate, hydrogen-enriched dialysate), and citrate-based anticoagulation. These approaches aim to suppress complement activation, reduce oxidative stress, and limit neutrophil-induced damage. Enhancing biocompatibility is crucial for reducing cardiovascular complications and improving outcomes in dialysis patients. Suppressing the innate immune response during dialysis may become a future cornerstone in extracorporeal blood purification therapy.
Keywords: NETosis; biocompatibility; complement; hemodialysis; interleukin-6; interleukin-8; microinflammation.
Conflict of interest statement
M.N. received honoraria for speaking at symposia from Nihon Trim. Co., Ltd. and holds a position on the advisory boards of Nihon Trim Co., Ltd. H.M. is conducting joint research with Nihon Trim Co., Ltd., and is receiving grant funding. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures

Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Hypothermia protects against ventilator-induced lung injury by limiting IL-1β release and NETs formation.Elife. 2025 Jun 24;14:RP101990. doi: 10.7554/eLife.101990. Elife. 2025. PMID: 40553503 Free PMC article.
-
Role of microRNAs in neutrophil extracellular trap formation and prevention: Systematic narrative review.Mol Cell Probes. 2024 Dec;78:101986. doi: 10.1016/j.mcp.2024.101986. Epub 2024 Oct 13. Mol Cell Probes. 2024. PMID: 39389272
-
Low dialysate sodium levels for chronic haemodialysis.Cochrane Database Syst Rev. 2024 Nov 5;11(11):CD011204. doi: 10.1002/14651858.CD011204.pub3. Cochrane Database Syst Rev. 2024. PMID: 39498822
-
IL-17A drives a fibroblast-neutrophil-NET axis to exacerbate immunopathology in the lung with diffuse alveolar damage.Front Immunol. 2025 Jun 11;16:1574246. doi: 10.3389/fimmu.2025.1574246. eCollection 2025. Front Immunol. 2025. PMID: 40568586 Free PMC article.
References
-
- Hörl W.H. Hemodialysis membranes: Interleukins, biocompatibility, and middle molecules. J. Am. Soc. Nephrol. 2002;13((Suppl. 1)):S62–S71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

