Identification of a PAK6-Mediated MDM2/p21 Axis That Modulates Survival and Cell Cycle Control of Drug-Resistant Stem/Progenitor Cells in Chronic Myeloid Leukemia
- PMID: 40650306
- PMCID: PMC12250115
- DOI: 10.3390/ijms26136533
Identification of a PAK6-Mediated MDM2/p21 Axis That Modulates Survival and Cell Cycle Control of Drug-Resistant Stem/Progenitor Cells in Chronic Myeloid Leukemia
Abstract
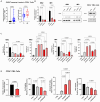
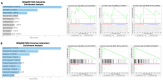
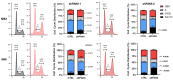
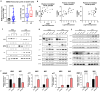
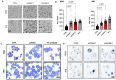
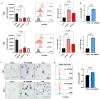
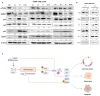
Chronic myeloid leukemia (CML) is a leading example of a malignancy where a molecular targeted therapy revolutionized treatment but has rarely led to cures. Overcoming tyrosine kinase inhibitor (TKI) drug resistance remains a challenge in the treatment of CML. We have recently identified miR-185 as a predictive biomarker where reduced expression in CD34+ treatment-naïve CML cells was associated with TKI resistance. We have also identified PAK6 as a target gene of miR-185 that was upregulated in CD34+ TKI-nonresponder cells. However, its role in regulating TKI resistance remains largely unknown. In this study, we specifically targeted PAK6 in imatinib (IM)-resistant cells and CD34+ stem/progenitor cells from IM-nonresponders using a lentiviral-mediated PAK6 knockdown strategy. Interestingly, the genetic and pharmacological suppression of PAK6 significantly reduced proliferation and increased apoptosis in TKI-resistant cells. Cell survivability was further diminished when IM was combined with PAK6 knockdown. Importantly, PAK6 inhibition in TKI-resistant cells induced cell cycle arrest in the G2-M phase and cellular senescence, accompanied by increased levels of DNA damage-associated senescence markers. Mechanically, we identified a PAK6-mediated MDM2-p21 axis that regulates cell cycle arrest and senescence. Thus, PAK6 plays a critical role in determining alternative cell fates in leukemic cells, and targeting PAK6 may offer a therapeutic strategy to selectively eradicate TKI-resistant cells.
Keywords: CML; DNA damage; Imatinib; PAK6; TKI resistance; TKI therapy; cell cycle; leukemic stem cells; senescence; small molecule inhibitors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Overexpression of miR-29a and miR-29b is involved in imatinib resistance via abrogated NF1 expression and increased ERK1/2 activation in chronic myeloid leukemia cells.Med Oncol. 2025 Jun 17;42(7):268. doi: 10.1007/s12032-025-02838-7. Med Oncol. 2025. PMID: 40526249
-
Nutrient-sensitizing drug repurposing screen identifies lomerizine as a mitochondrial metabolism inhibitor of chronic myeloid leukemia.Sci Transl Med. 2024 Jun 12;16(751):eadi5336. doi: 10.1126/scitranslmed.adi5336. Epub 2024 Jun 12. Sci Transl Med. 2024. PMID: 38865484
-
KF1601, a dual inhibitor of BCR::ABL1 and FLT3, overcomes drug resistance in FLT3+ blast phase chronic myeloid leukemia.Mol Cancer. 2025 Apr 14;24(1):114. doi: 10.1186/s12943-025-02292-z. Mol Cancer. 2025. PMID: 40229844 Free PMC article.
-
Potential therapeutic targets in chronic myeloid leukemia.Med Oncol. 2025 Jul 17;42(8):344. doi: 10.1007/s12032-025-02895-y. Med Oncol. 2025. PMID: 40676443 Review.
-
Treatment-Free Remission in Chronic Myeloid Leukemia: Revisiting the "W" Questions.Eur J Haematol. 2025 Sep;115(3):218-231. doi: 10.1111/ejh.70000. Epub 2025 Jun 23. Eur J Haematol. 2025. PMID: 40550748 Free PMC article. Review.
References
-
- Zhou L.L., Zhao Y., Ringrose A., DeGeer D., Kennah E., Lin A.E.-J., Sheng G., Li X.-J., Turhan A., Jiang X. AHI-1 Interacts with BCR-ABL and Modulates BCR-ABL Transforming Activity and Imatinib Response of CML Stem/Progenitor Cells. J. Exp. Med. 2008;205:2657–2671. doi: 10.1084/jem.20072316. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

