Evaluation of Torquetenovirus (TTV) Particle Integrity Utilizing PMAxx™
- PMID: 40650319
- PMCID: PMC12250055
- DOI: 10.3390/ijms26136542
Evaluation of Torquetenovirus (TTV) Particle Integrity Utilizing PMAxx™
Abstract
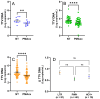
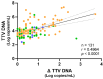
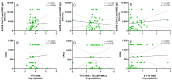
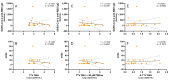
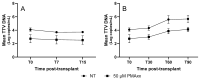
Torquetenovirus (TTV) is a ubiquitous, non-pathogenic DNA virus that has been suggested as a biomarker of immune competence, with the viral load correlating with the level of immunosuppression. However, by detecting non-intact viral particles, standard PCR-based quantification may overestimate the TTV viremia. To improve the clinical relevance of TTV quantification, in this study, we investigated the use of PMAxx™, a virion viability dye that selectively blocks the amplification of compromised virions. Serum samples from 10 Hepatitis C Virus-positive (HCV+) individuals, 81 liver transplant recipients (LTRs), and 40 people with HIV (PWH) were treated with PMAxx™ and analyzed for TTV DNA loads by digital droplet PCR (ddPCR). Furthermore, anti-SARS-CoV-2 IgG levels and neutralizing antibody (nAbs) titers were measured post-COVID-19 vaccination. Using ddPCR, the PMAxx™ treatment significantly reduced the TTV DNA levels in all the groups (mean reduction: 0.66 Log copies/mL), indicating the abundant presence of non-intact, circulating viral genomes. However, correlations between TTV DNA and SARS-CoV-2 IgG or nAbs were weak or absent in both PMAxx™-treated and untreated samples. These findings suggest that while PMAxx™ enhanced the specificity of TTV quantification, it did not improve the predictive value of TTV viremia at assessing vaccine-induced humoral responses.
Keywords: COVID-19 vaccination; Torquetenovirus; genome quantification; propidium monoazide (PMA).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Anti-SARS-CoV-2 total immunoglobulin and neutralising antibody responses in healthy blood donors throughout the COVID-19 pandemic: a longitudinal observational study.Swiss Med Wkly. 2024 Jul 1;154:3408. doi: 10.57187/s.3408. Swiss Med Wkly. 2024. PMID: 39137369
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
The effect of early COVID-19 treatment with convalescent plasma on antibody responses to SARS-CoV-2.Microbiol Spectr. 2025 Jul;13(7):e0300624. doi: 10.1128/spectrum.03006-24. Epub 2025 Jun 9. Microbiol Spectr. 2025. PMID: 40488473 Free PMC article. Clinical Trial.
-
Immune response induced by the recombinant novel coronavirus vaccine (Adenovirus type 5 vector) (Ad5-nCoV) in persons living with HIV (PLWH).PLoS One. 2025 Jun 11;20(6):e0312893. doi: 10.1371/journal.pone.0312893. eCollection 2025. PLoS One. 2025. PMID: 40498774 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

