A specifically designed multi-biotic reduces uremic toxin generation and improves kidney function
- PMID: 40650408
- PMCID: PMC12258176
- DOI: 10.1080/19490976.2025.2531202
A specifically designed multi-biotic reduces uremic toxin generation and improves kidney function
Abstract
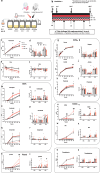
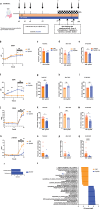
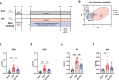
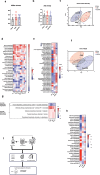
Chronic kidney disease (CKD) is characterized by accumulation of uremic toxins (UTs), such as p-cresyl sulfate and indoxyl sulfate, generated through the transformation of tyrosine and tryptophan by the gut microbiota. Using an ex vivo Simulator of the Human Intestinal Microbial Ecosystem (SHIME) colonized with fecal samples from eight CKD patients or nine healthy volunteers, a higher bacterial generation of p-cresol and indoles post-amino acid enrichment, as well lower basal butyrate levels, in the feces of CKD patients were found. Through in silico data mining, we selected a probiotic strain lacking the capacity to produce UT, i.e. without genes for tryptophanase, tyrosinase and urease. In vitro, we confirmed the potential of cellobiose as a prebiotic supporting the growth of this strain. We further designed a novel specific multi-biotic for CKD (SynCKD) [containing a probiotic Lactobacillus johnsonii NCC533, a prebiotic (1% cellobiose), and a postbiotic (1% short and medium chain triglycerides C4-C8, a source of butyrate)]. SynCKD effectively curtailed UT precursor generation ex vivo. The in vivo efficacy of SynCKD (and the synergic effect) was established in two uremic rodent models, demonstrating lower plasma levels of UTs and enhancing kidney function after 6-8 weeks of treatment. These effects were linked to better gut microbial ecology. Metagenomic analysis revealed reduced microbial genes for tryptophan/tyrosine degradation. This study lays the foundation for SynCKD as a potential therapy to mitigate CKD progression.
Keywords: Multi-biotic; chronic kidney disease; gut microbiota; uremic toxins.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest, other than Nestlé. AB, BB, CP, CB, GG, and MJ have no other conflicts of interest; HV has received speaker fees from Sanofi; DF has received lecture fees and travel support from Vifor, Dr Schär, B. Braun, Fresenius Kabi, Astellas, AstraZeneca, and Lilly; LK has received grants from Fresenius Kabi, Nestlé, Lallemand, AstraZeneca, and consultancy or speaker fees or travel support from AstraZeneca, Lilly, Baxter, Bayer, and Fresenius Kabi; PD and JN are employees of Nestlé Health Science and conducted the research within this framework. SD and YF were employees of Nestlé Research and conducted the research within this framework.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
