Programmable probiotic consortium employ an oleic acid-inducible system to sense and degrade cholesterol in high-fat diet mice
- PMID: 40650420
- PMCID: PMC12258189
- DOI: 10.1080/19490976.2025.2531198
Programmable probiotic consortium employ an oleic acid-inducible system to sense and degrade cholesterol in high-fat diet mice
Abstract
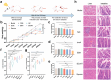
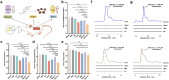
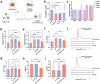
High cholesterol is a major risk factor for cardiovascular disease (CVD), and current treatment strategies primarily focus on inhibiting cholesterol synthesis and reducing cholesterol absorption. Engineered synthetic consortia are emerging as a promising alternative for lowering cholesterol level and improving cardiovascular health. In this study, Escherichia coli Nissle 1917 (EcN) was engineered to express three genes -IsmA, BSH, and BCoAT, individually. The IsmA-expressing strain achieved a 27.95% reduction in cholesterol, the BSH-expressing strain exhibited a bile salt hydrolase activity of 6.14 U/mL, and the butyric acid production of the BCoAT-expressing strain was 5.81 mmol/L. The synthetic consortium (IsmA-, BSH-, and BCoAT-expressing strains) reduced serum cholesterol level by 43.65% in mice fed high-fat diet. Further, an oleic acid-inducible system was incorporated into the synthetic consortium (GR-Syncon). In contrast to Syncon, GR-Syncon is capable of recognizing fatty acid levels and expressing target genes exclusively in high-fat environments. In high-fat diet mice, administration of GR-Syncon resulted in a 27.60% reduction in serum cholesterol, downregulated the expression of cholesterol synthase HMGCR (31.97%), and upregulated cholesterol hydroxylase Cyp7a1 (86.74%), optimizing lipid metabolism. GR-Syncon also down-regulated inflammatory cytokines (IL-1β, IL-10 and TNF-α), repair liver damage, improved intestinal permeability and maintained the stability of gut microbiota in high-fat diet mice. This study highlights the potential of engineered synthetic consortia as a sustainable and targeted strategy for managing cholesterol and treating CVD.
Keywords: Synthetic biology; cholesterol; engineered probiotics; high-fat diet; lipid metabolism.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



Similar articles
-
Study on the modulation of kidney and liver function of rats with diabetic nephropathy by Huidouba through metabolomics.J Ethnopharmacol. 2025 Jul 24;351:120136. doi: 10.1016/j.jep.2025.120136. Epub 2025 Jun 11. J Ethnopharmacol. 2025. PMID: 40513925
-
Modulation of Gut Microbial Composition by Lactobacillus delbrueckii subsp. lactis CKDB001 Supplementation in a High-Fat-Diet-Induced Obese Mice.Nutrients. 2025 Jul 7;17(13):2251. doi: 10.3390/nu17132251. Nutrients. 2025. PMID: 40647355 Free PMC article.
-
Lactiplantibacillus plantarum dfa1 reduces obesity caused by a high carbohydrate diet by modulating inflammation and gut microbiota.Sci Rep. 2025 Jul 10;15(1):24801. doi: 10.1038/s41598-025-10435-x. Sci Rep. 2025. PMID: 40634535 Free PMC article.
-
Probiotics for the prevention of pediatric antibiotic-associated diarrhea.Cochrane Database Syst Rev. 2011 Nov 9;(11):CD004827. doi: 10.1002/14651858.CD004827.pub3. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2015 Dec 22;(12):CD004827. doi: 10.1002/14651858.CD004827.pub4. PMID: 22071814 Updated.
-
The Therapeutic Effects of Probiotic on Systemic Lupus Erythematosus in Lupus Mice Models: A Systematic Review.Probiotics Antimicrob Proteins. 2025 Feb;17(1):35-50. doi: 10.1007/s12602-024-10297-1. Epub 2024 May 28. Probiotics Antimicrob Proteins. 2025. PMID: 38806966
References
-
- Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143(8):e254–19. doi: 10.1161/CIR.0000000000000950. - DOI - PubMed
-
- Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
