Menstrual cycle influence on skeletal muscle mitochondrial respiration in humans
- PMID: 40650425
- PMCID: PMC12254574
- DOI: 10.14814/phy2.70458
Menstrual cycle influence on skeletal muscle mitochondrial respiration in humans
Abstract
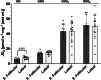
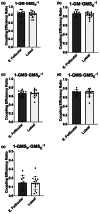
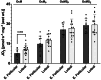
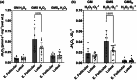
The menstrual cycle influences function in various tissues in the body. We sought to determine if menstrual cycle phase could influence mitochondrial function in skeletal muscle in females. Twenty-nine females with regular menstrual cycles were randomized to have a vastus lateralis muscle biopsy during either the early follicular or luteal phase. High-resolution respirometry was used to determine mitochondrial respiration on permeabilized muscle fibers. Glutamate/malate LEAK respiration was significantly higher during the luteal phase compared to the early follicular phase. Glutamate/malate/succinate LEAK respiration was the same during both menstrual cycle phases, as was maximal coupled and uncoupled respiration. There were no differences in fatty acid-supported respiration. The fatty acid-supported coupling efficiency ratios of 1-OcM (octanoylcarnitine/malate) LEAK over maximal coupled respiration and 1-OcM LEAK over maximal uncoupled respiration were both significantly higher in mitochondria from the early follicular phase than in the luteal phase. Mitochondrial H2O2 emission (glutamate/malate/succinate supported) was significantly increased in muscle from the early follicular phase. We detected no differences in mitochondrial content using citrate synthase activity between phases of the menstrual cycle. Collectively, our observations demonstrate a limited influence of the menstrual cycle on certain measures of submaximal respiration, coupling efficiencies, and H2O2 emission.
Keywords: menstrual cycle phase; mitochondrial respiration; skeletal muscle.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
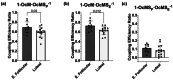
References
-
- Benton, C. R. , Nickerson, J. G. , Lally, J. , Han, X. X. , Holloway, G. P. , Glatz, J. F. , Luiken, J. J. , Graham, T. E. , Heikkila, J. J. , & Bonen, A. (2008). Modest PGC‐1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. The Journal of Biological Chemistry, 283, 4228–4240. - PubMed
-
- Braun, B. , & Horton, T. (2001). Endocrine regulation of exercise substrate utilization in women compared to men. Exercise and Sport Sciences Reviews, 29, 149–154. - PubMed
-
- Brown, J. L. , Rosa‐Caldwell, M. E. , Lee, D. E. , Blackwell, T. A. , Brown, L. A. , Perry, R. A. , Haynie, W. S. , Hardee, J. P. , Carson, J. A. , Wiggs, M. P. , Washington, T. A. , & Greene, N. P. (2017). Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour‐bearing mice. Journal of Cachexia, Sarcopenia and Muscle, 8, 926–938. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

