ACSS2-Mediated Histone H4 Lysine 12 Crotonylation (H4K12cr) Alleviates Colitis via Enhancing Transcription of CLDN7
- PMID: 40650658
- PMCID: PMC12376547
- DOI: 10.1002/advs.202500461
ACSS2-Mediated Histone H4 Lysine 12 Crotonylation (H4K12cr) Alleviates Colitis via Enhancing Transcription of CLDN7
Abstract
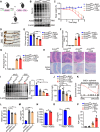
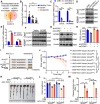
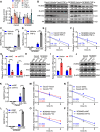
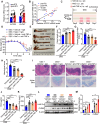
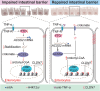
Histone lysine crotonylation (Kcr), a highly conserved posttranslational modification, plays critical roles in various biological processes. Nevertheless, the dynamic alterations and functions of histone Kcr in inflammatory bowel disease (IBD) remain poorly explored. Herein, a notable decrease of both Pan-Kcr and ACSS2 (acyl-CoA synthetase short-chain family member 2), the key enzyme for crotonyl-CoA generation, is revealed in inflamed intestinal epithelial cells. Genetic or pharmacological inhibition of ACSS2 dramatically impairs mouse intestinal barrier integrity and exacerbates colitis. Mechanistically, ACSS2-mediated histone H4 lysine 12 crotonylation (H4K12cr) upregulates CLDN7 expression to fortify intestinal epithelial barrier, which can be augmented by crotonate supplementation. Furthermore, tumor necrosis factor-α (TNF-α) is revealed to enhance the m6A modification of ACSS2 mRNA, consequently destabilizing and downregulating ACSS2. Combinational therapy involving anti-TNF-α and crotonate can significantly ameliorate colitis. Overall, ACSS2-mediated H4K12cr emerges as a pivotal modulator governing intestinal barrier function during IBD progression.
Keywords: ACSS2; histone lysine crotonylation; inflammatory bowel disease; intestinal barrier.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ng S. C., Shi H. Y., Hamidi N., Underwood F. E., Tang W., Benchimol E. I., Panaccione R., Ghosh S., Wu J. C. Y., Chan F. K. L., Sung J. J. Y., Kaplan G. G., Lancet 2017, 390, 2769. - PubMed
-
- Chang J. T., N. Engl. J. Med. 2020, 383, 2652. - PubMed
-
- Villablanca E. J., Selin K., Hedin C. R. H., Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 493. - PubMed
MeSH terms
Substances
Grants and funding
- 82370675/National Natural Science Foundation of China
- 82470570/National Natural Science Foundation of China
- 82303928/National Natural Science Foundation of China
- 2024B03J0211/Key Research and Development Program of Guangzhou
- 2024A1515012813/Guangdong Basic and Applied Basic Research Foundation
- 2022A1515111043/Guangdong Basic and Applied Basic Research Foundation
- 2024A1515013072/Guangdong Basic and Applied Basic Research Foundation
- 23xkjc023/Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- 23qnpy147/Fundamental Research Funds for the Central Universities, Sun Yat-sen University
- 2022YFA1304000/National Key R&D Program of China
- 2020B1111170004/the program of Guangdong Provincial Clinical Research Center for Digestive Diseases
- 2022JBGS01/the "Jie Bang Gua Shuai" Program from The Sixth Affiliated Hospital, Sun Yat-sen University
- 2023A04J1820/Guangzhou Basic and Applied Basic Research Foundation
- 2021276/the Starting Funding of Faculty from Sun Yat-sen University
- 20242BAB25537/Jiangxi Provincial Natural Science Foundation
- 2024E02063/Xinjiang Uyghur Autonomous Regional Collaborative Innovation Special Project-Science and Technology Assistance to Xinjiang
- 2024D01C05/Xinjiang Uyghur Autonomous Regional Natural Science Foundation
- A2402002/Shenzhen Medical Research Fund
- the National Key Clinical Discipline
LinkOut - more resources
Full Text Sources
