Real-world effectiveness and safety of first-line chemoimmunotherapy combinations in metastatic non-small cell lung cancer with programmed death ligand-1 < 50%: results from an Italian observational study
- PMID: 40650694
- PMCID: PMC12255629
- DOI: 10.1007/s00262-025-04125-w
Real-world effectiveness and safety of first-line chemoimmunotherapy combinations in metastatic non-small cell lung cancer with programmed death ligand-1 < 50%: results from an Italian observational study
Abstract
Introduction: This multi-center, observational cohort study aimed to evaluate the real-world effectiveness and safety of two first-line chemoimmunotherapy combinations-pembrolizumab plus chemotherapy and nivolumab/ipilimumab plus chemotherapy-in patients with metastatic non-small cell lung cancer (NSCLC) and programmed death ligand-1 (PD-L1) expression < 50%.
Patients and methods: The primary objectives were progression-free survival (PFS) and overall survival (OS) in the overall population. Secondary objectives included the incidence of chemotherapy-related and immune-related adverse events (irAEs).
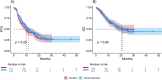
Results: A total of 495 patients were enrolled, with 348 (70.3%) receiving pembrolizumab plus chemotherapy and 147 (29.7%) treated with nivolumab/ipilimumab plus chemotherapy. Overall, median follow-up was 11 (95% CI: 10.2 12.2) months. The median PFS was 10.9 months (95% CI: 9.6-13), and the median OS was 21.1 months (95% CI: 16.8-NR) in the overall population. In multivariable analysis, ECOG PS ≥ 2, PD-L1 expression < 1%, squamous histology, baseline steroid use, and the presence of CNS, bone, or liver metastases were significantly associated with shorter survival. No significant differences were observed between the pembrolizumab and nivolumab/ipilimumab cohorts in terms of PFS (11.83 vs. 9.83 months; HR 0.86, 95% CI: 0.67-1.11, p = 0.3) or OS (21.3 vs. 20.6 months; HR 1.03, 95% CI: 0.76-1.39, p = 0.9). Chemotherapy-related adverse events were more frequent in the pembrolizumab cohort, whereas irAEs were more common in the nivolumab/ipilimumab cohort.
Conclusion: In this real-world study, chemoimmunotherapy combinations demonstrated manageable toxicity profiles, with effectiveness comparable to that reported in pivotal phase 3 randomized trials. Pembrolizumab and nivolumab/ipilimumab showed similar real-world effectiveness but significantly different toxicity profiles.
Keywords: Chemotherapy; Ipilimumab; NSCLC; Nivolumab; PD-L1; Pembrolizumab.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Alessandro Inno: honoraria/advisory board roles from Amgen, Astra Zeneca, Merck Sharp-Dome, Novartis, Roche. Carlo Genova: honoraria/advisory board roles from Amgen, Astra Zeneca, Bristol-Myers-Squibb, Daiichi-Sankyo, Eli Lilly, Merck-Sharp-Dohme, Novartis, Pierre Fabre, Regeneron, Roche, Takeda. The other authors declare no conflicts of interest.
Figures
References
-
- Hendriks LE, Kerr KM, Menis J, Mok TS, Nestle U, Passaro A, Peters S, Planchard D, Smit EF, Solomon BJ, Veronesi G, Reck M, ESMO Guidelines Committee (2023) Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 34(4):358–376. 10.1016/j.annonc.2022.12.013 - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19):1823–1833. 10.1056/NEJMoa1606774 - PubMed
-
- Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov O, Clingan P, Sriuranpong V, Rizvi N, Gao B, Li S, Lee S, McGuire K, Chen CI, Makharadze T, Paydas S, Nechaeva M, Seebach F, Weinreich DM, Yancopoulos GD, Gullo G, Lowy I, Rietschel P (2021) Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 397(10274):592–604. 10.1016/S0140-6736(21)00228-2 - PubMed
-
- Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR (2020) Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 383(14):1328–1339. 10.1056/NEJMoa1917346 - PubMed
-
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, KEYNOTE-189 Investigators (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092. 10.1056/NEJMoa1801005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials