Efficacy and Safety of Biosimilar CT-P47 Versus Reference Tocilizumab: 1-Year Results of a Randomised, Active-Controlled, Double-Blind, Phase III Study in Patients with Rheumatoid Arthritis
- PMID: 40650731
- PMCID: PMC12307557
- DOI: 10.1007/s40261-025-01453-8
Efficacy and Safety of Biosimilar CT-P47 Versus Reference Tocilizumab: 1-Year Results of a Randomised, Active-Controlled, Double-Blind, Phase III Study in Patients with Rheumatoid Arthritis
Abstract
Background and objective: This phase III study conducted in 22 centres in Poland assessed the efficacy equivalence of candidate tocilizumab biosimilar, CT-P47, and European Union-approved reference tocilizumab (r-TCZ) in rheumatoid arthritis. We report 1-year data, including switching from r-TCZ to CT-P47.
Methods: This active-controlled, double-blind, multicentre trial randomised (1:1) adults (aged 18-75 years) with moderate-to-severe rheumatoid arthritis diagnosed for ≥ 24 weeks and treated with methotrextate for ≥ 12 weeks before the first study drug administration, to receive CT-P47 or r-TCZ every 4 weeks (8 mg/kg, intravenous) up to week 20. At week 24, those on CT-P47 continued maintenance treatment; those on r-TCZ were re-randomised (1:1) to continue r-TCZ (r-TCZ maintenance) or to switch to CT-P47 (CT-P47 switched) until week 48 (Treatment Period 2). After week 48, patients were followed up until week 52 (end of study). Efficacy, pharmacokinetics, safety and immunogenicity were evaluated.
Results: In Treatment Period 2, 225 patients continued CT-P47 maintenance, 109 continued r‑TCZ maintenance and 110 switched to CT-P47. During Treatment Period 2, efficacy findings were comparable between groups. At week 52, the mean change from baseline in Disease Activity Score in 28 joints-erythrocyte sedimentation rate was - 4.279, - 4.231 and - 4.376 in the CT-P47 maintenance, r-TCZ maintenance and CT-P47 switched groups, respectively. Joint damage progression over 1 year was minimal in all groups. Drug serum concentrations were relatively consistent throughout Treatment Period 2. The safety profile and antidrug antibody-positive conversion rate (< 5% in each group) were similar between groups.
Conclusions: Week 52 results show maintained efficacy after switching from r-TCZ to CT-P47, and comparable efficacy, pharmacokinetics, safety and immunogenicity of CT-P47 versus r-TCZ over 1 year of treatment.
Clinical trial registration: NCT05489224, 24 July 2022.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Medical University of Vienna. This study was funded by Celltrion, Inc. (Incheon, Republic of Korea). The study sponsor, Celltrion, Inc. (Incheon, Republic of Korea), played a role in the study design, data collection and analysis, decision to publish and preparation of the manuscript. Conflict of Interest/Competing Interests: Gerd Burmester has received honoraria for consulting and lectures from Celltrion, Inc., Chugai, Fresenius and Sanofi. Marek Krogulec has received support for meeting attendance from Accord, Egis, Medac and Sandoz. Sławomir Jeka has received consulting fees from AbbVie, Celltrion, Inc., Eli Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Sanofi and UCB; and payments or honoraria from AbbVie, Celltrion, Inc., Eli Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Sanofi and UCB. Rafał Wojciechowski has received speaker fees from Eli Lilly, Janssen, Novartis, SOBI and UCB; support for meeting attendance from AbbVie; and is involved in the leadership of the Polish Rheumatology Society. Magdalena Krajewska-Włodarczyk has received payments or honoraria from AbbVie, Boehringer Ingelheim, Eli Lilly, Janssen, Medac, MSD, Novartis, Pfizer, Sandoz, SOBI and UCB; and support for meeting attendance from AbbVie, Medac, Novartis, Pfizer and SOBI. Paweł Hrycaj has received research grants from Celltrion, Inc. SungHyun Kim is an employee of Celltrion, Inc. JeeHye Suh, GoEun Yang, YunAh Kim, YooBin Jung and GaHee Park are employees of Celltrion, Inc., and hold stocks in Celltrion, Inc. Josef S. Smolen has received payments to his institution from AbbVie, AstraZeneca, Eli Lilly, Novartis, Galapagos and Roche; personal fees from AbbVie, Amgen, Ananda, Astro, BMS, Celltrion, Inc., Chugai, Eli Lilly, Gilead, Immunovant, MSD, Novartis, Pfizer, Roche, R-Pharma, Samsung, Sanofi and UCB; payments or honoraria from Eli Lilly; and support for meeting attendance from Eli Lilly. Jakub Trefler, Artur Racewicz, Janusz Jaworski, Agnieszka Zielińska, Katarzyna Kolossa, Anna Dudek and Piotr Adrian Klimiuk have no conflicts of interest that are directly relevant to the content of this article. Ethics Approval: The study was conducted in accordance with the Declaration of Helsinki (and Good Clinical Practice Guideline). All national, state, and local laws or regulations were followed. Prior to study initiation, the study protocol, informed consent form, advertisements used for the recruitment of participants and any other written materials provided to participants were approved by the institutional review board. This study involves human participants and was approved by the Institutional Review Board, Komisja Bioetyczna przy OIL w Białymstoku, ul. Świętojańska 7, 15-082 Białystok, Poland, and the Ethics Committee at the Regional Medical Chamber with its seat in Białystok, 15-082 Białystok, ul. Świętojańska 7, Poland (reference number: 43/2022/VIII). Consent to Participate: Written informed consent was obtained from all patients prior to enrolment. Consent for Publication: Not applicable. Availability of Data and Material: All data relevant to the study are included in the article or uploaded as supplementary information. Code Availability: Not applicable. Authors’ Contributions: GB, SHK, JHS, GEY, YAK, YBJ, GHP and JSS contributed to the study design. JT, AR, JJ, AZ, MK, SJ, RW, KK, AD, MKW, PH, PAK, SHK, GEY, YBJ and GHP contributed to the data collection. All authors contributed to the data analysis or interpretation, critically reviewed and critically revised the manuscript, approved the final version for publication, and agree to be accountable for the accuracy and integrity of the work.
Figures
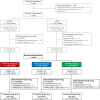

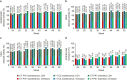
References
-
- European Medicines Agency. RoActemra summary of product characteristics. 2024. https://www.ema.europa.eu/en/documents/product-information/roactemra-epa.... Accessed 5 Sept 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

