Stem Cell for Cancer Immunotherapy: Current Approaches and Challenges
- PMID: 40650799
- PMCID: PMC12408727
- DOI: 10.1007/s12015-025-10933-5
Stem Cell for Cancer Immunotherapy: Current Approaches and Challenges
Abstract
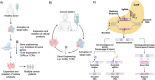
Stem cell-based immunotherapy represents a groundbreaking advancement in cancer treatment, leveraging the immune system's inherent capacity to target and eradicate cancer cells. This review explores some of the examples of stem cells used in cancer immunotherapy, including hematopoietic, mesenchymal, and induced pluripotent stem cells (IPSCs). It also describes stem cell functionalities like modifying tumor microenvironment (TME) and developing engineered immune cells like chimeric antigen receptor (CAR)-T cells and natural killer (NK) cells. Additionally, the clinical applications of stem cells for improving cancer immunotherapies and delivering drugs directly to solid tumors are discussed. However, several challenges limit the effectiveness of stem cell technology, including safety risks, tumor avoidance by the immune system, and regulatory protocols as well as manufacturing barriers. This article reviews current advancements to overcome these challenges, such as CRISPR-based gene editing and targeted drug delivery systems and provides an outlook on emerging trends, such as the progress of personalized stem cell therapies and the increasing effectiveness of treatment by combining them with other cancer treatments.
Keywords: CAR-T cells; CRISPR; Cancer; Immunotherapy; Microenvironment; Stem cells.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interest: The authors affirm that they do not have any competing interests.
Figures









References
-
- International Agency for Research on Cancer (IARC). (2024). Global Cancer Observatory: Cancer Tomorrow. International Agency for Research on Cancer (IARC).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

