A transfer RNA methyltransferase with an unusual domain composition catalyzes 2'-O-methylation at position 6 in tRNA
- PMID: 40650981
- PMCID: PMC12255305
- DOI: 10.1093/nar/gkaf579
A transfer RNA methyltransferase with an unusual domain composition catalyzes 2'-O-methylation at position 6 in tRNA
Abstract
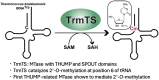
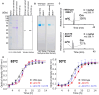
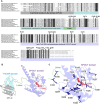
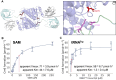
Thermococcus kodakarensis tRNATrp contains 2'-O-methylcytidine at position 6 (Cm6). However, the tRNA methyltransferase responsible for the modification has not been identified. Using comparative genomics, we predicted TK1257 as a candidate gene for the modification enzyme. Biochemical and mass spectrometry studies of purified recombinant TK1257 gene product demonstrated that it possesses a tRNA methyltransferase activity for Cm6 formation. This protein has a highly unusual composition of domains, containing N-terminal ferredoxin-like, SPOUT catalytic, and THUMP domains. Previous to this study, all known THUMP-related tRNA methyltransferases were shown to contain a Rossmann fold catalytic domain and the nucleosides they produced were N2-methylguanosine and/or N2, N2-dimethylguanosine. Therefore, our findings extend the knowledge of architecture of tRNA methyltransferases. We named the TK1257 gene product TrmTS and showed that it can synthesize Am6 and Um6 as well as Cm6. A trmTS gene deletion strain showed slight growth retardation at high temperatures. Site-directed mutagenesis studies revealed catalytically and structurally important amino acid residues in TrmTS and identified a TrmTS-specific linker that is structurally essential. We revealed that TrmTS recognizes the 3'-CCA terminus of tRNA but does not require the three-dimensional structure of tRNA for its activity. Finally, we constructed a model of the binding between TrmTS and tRNA.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Transfer RNA Recognition Mechanism of Thermoplasma acidophilum Trm56, a SPOUT tRNA Methyltransferase that Possesses an Unusually Long C-terminal Region.J Mol Biol. 2025 Oct 1;437(19):169328. doi: 10.1016/j.jmb.2025.169328. Epub 2025 Jul 14. J Mol Biol. 2025. PMID: 40664129
-
Functional redundancy of ubiquitin-like sulfur-carrier proteins facilitates flexible, efficient sulfur utilization in the primordial archaeon Thermococcus kodakarensis.mBio. 2024 Aug 14;15(8):e0053424. doi: 10.1128/mbio.00534-24. Epub 2024 Jul 8. mBio. 2024. PMID: 38975783 Free PMC article.
-
Structural and functional analyses of the archaeal tRNA m2G/m22G10 methyltransferase aTrm11 provide mechanistic insights into site specificity of a tRNA methyltransferase that contains common RNA-binding modules.Nucleic Acids Res. 2016 Jul 27;44(13):6377-90. doi: 10.1093/nar/gkw561. Epub 2016 Jun 20. Nucleic Acids Res. 2016. PMID: 27325738 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

