Notch activity is modulated by the aGPCR Latrophilin binding the DSL ligand in C. elegans
- PMID: 40651965
- PMCID: PMC12255766
- DOI: 10.1038/s41467-025-61730-0
Notch activity is modulated by the aGPCR Latrophilin binding the DSL ligand in C. elegans
Abstract
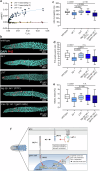
The Notch pathway is a highly conserved signaling cascade across metazoans that regulates numerous physiological processes, including cell proliferation, differentiation, and fate determination. Given its fundamental roles, the pathway is tightly regulated by diverse molecules through multiple mechanisms. Here, we identify the Adhesion GPCR latrophilin (LPHN/ADGRL) as a positive modulator of Notch signaling, which increases Notch receptor activation and the translocation of its intracellular domain into the nucleus. Physiologically, this latrophilin role is crucial for balancing the number of proliferating cells in the gonadal stem cell niche of the nematode C. elegans. In silico, in vitro, and in vivo analyses demonstrate that the C. elegans latrophilin homolog LAT-1 directly interacts with the DSL protein/Notch ligand LAG-2 on the same cell. This interaction is mediated by LAT-1's conserved GAIN and the RBL domain. Importantly, the modulatory effect depends solely on the receptor's N terminus and is independent of G protein signaling. Finally, we explore the implications of this fine-tuning of Notch signaling by an aGPCR.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Gupte, J. et al. Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett.586, 1214–1219 (2012). - PubMed
MeSH terms
Substances
Grants and funding
- P40 OD010440/OD/NIH HHS/United States
- CRC 1423/2; 421152132; C04/Deutsche Forschungsgemeinschaft (German Research Foundation)
- FOR2149; 246212759; P04/Deutsche Forschungsgemeinschaft (German Research Foundation)
- CRC1423/2; 421152132; Z04/Deutsche Forschungsgemeinschaft (German Research Foundation)
- CRC1423/2; 421152132; B03/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SPP2363; 460865652/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SCADS24B/Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research)
- Humboldt Professorship/Alexander von Humboldt-Stiftung (Alexander von Humboldt Foundation)
- FOR2149; 246212759; P02/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Research Materials

