Endogenous tyrosinase-catalyzed therapeutics
- PMID: 40651969
- PMCID: PMC12255739
- DOI: 10.1038/s41467-025-61799-7
Endogenous tyrosinase-catalyzed therapeutics
Abstract
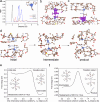
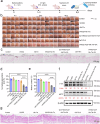
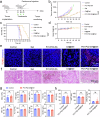
Tyrosinase (TYR) catalyzes the two initial steps of melanin synthesis from tyrosine in various organisms. However, overproduction, accumulation, and abnormal reduction of melanin can lead to severe diseases, particularly skin diseases, which makes tyrosinase a significant endogenous target in developing therapeutics to treat melanin-associated disorders. Herein, we devise a TYR-based in situ catalytic platform that can generate drugs intracellularly through an endogenous copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction. By taking advantage of the potent catalytic activity of TYR that is mechanistically validated by ab initio molecular dynamics (AIMD) theoretical calculation and experimental catalysis performance, we develop a TYR-catalyzed in-situ formed proteolysis-targeting chimeras (PROTACs) to degrade intracellular TYR protein to decrease melanin synthesis for treating hyperpigmentation and a TYR-catalyzed in-situ activated prodrug strategy to overcome drug resistance for melanoma therapy. In male mouse models, we show that this TYR-catalyzed therapeutics could efficiently alleviate skin hyperpigmentation within 48 h as well as resensitize the drug-resistant melanoma cells to chemotherapeutics to control tumor growth. Together, we offer an integrative platform to leverage the catalytic activity of endogenous TYR to generate therapeutics through in situ bioorthogonal chemistry for treating melanin-associated skin diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Vaezi, M. Structure and inhibition mechanism of some synthetic compounds and phenolic derivatives as tyrosinase inhibitors: review and new insight. J. Biomol. Struct. Dyn.41, 4798–4810 (2023). - PubMed
-
- Pomerantz, S. H. & Li, J. P. C. Tyrosinase in the skin of albino hamsters and mice. Nature252, 241–243 (1974). - PubMed
-
- Klabunde, T., Eicken, C., Sacchettini, J. C. & Krebs, B. Crystal structure of a plant catechol oxidase containing a dicopper center. Nat. Struct. Biol.5, 1084–1090 (1998). - PubMed
MeSH terms
Substances
Grants and funding
- R01CA288851/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- 1R01EB035992/U.S. Department of Health & Human Services | NIH | National Institute of Biomedical Imaging and Bioengineering (NIBIB)
- RSG-23-1140821-01-ET/American Cancer Society (American Cancer Society, Inc.)
LinkOut - more resources
Full Text Sources

