Hyperglycemia-induced mitochondrial abnormalities in autonomic neurons via the RAGE axis
- PMID: 40652096
- PMCID: PMC12255778
- DOI: 10.1038/s41598-025-10933-y
Hyperglycemia-induced mitochondrial abnormalities in autonomic neurons via the RAGE axis
Abstract
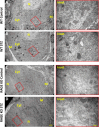
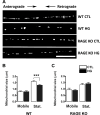
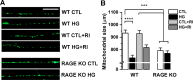
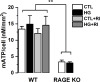
Diabetic peripheral neuropathy is characterized by mitochondrial dysfunction, including suboptimal respiration, impaired calcium homeostasis, and accumulation of reactive oxygen species (ROS). Hyperglycemia drives excessive electron donation to the mitochondria, resulting in damaging ROS levels. In peripheral neurons, hyperglycemia also leads to the formation of advanced glycation end-products (AGEs), which, through their membrane receptor (RAGE), trigger autonomic malfunction in diabetes. However, it remains unclear whether RAGE is required to induce mitochondrial abnormalities under hyperglycemia. Thus, we first investigated mitochondrial morphology in autonomic ganglia (superior cervical ganglion, SCG) from streptozotocin (STZ)-induced diabetic mice and found an enhanced proportion of swollen mitochondria with disrupted cristae in wild-type (WT) diabetic mice, but not in RAGE knock-out (KO) diabetic mice. Next, we exposed cultured SCG neurons to high glucose and found fragmentation and an imbalanced traffic represented by an increased proportion of only anterograde moving mitochondria in neurons from WT, but such imbalance was not observed in neurons from RAGE KO mice. Treating WT neurons with the non-membrane permeable RAGE selective inhibitor FPS-ZM1, did not prevent fragmentation, although a non-significant restoring trend was observed. Furthermore, ATP production was unaffected by exposure to high glucose in neurons for WT, and remained unchanged by incubation in FPS-ZM1. Interestingly, neurons from RAGE KO mice had significantly less ATP produced in all conditions than those from WT mice. Lastly, we found RAGE protein in enriched mitochondrial fractions from nerve growth factor (NGF)-transformed PC12 (PC12NGF) cells, as well as the colocalization of RAGE with a mitochondrial marker in cultured SCG neurons. Therefore, our data support that RAGE mediates mitochondrial damage in autonomic neurons under hyperglycemic conditions.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: This study was performed in line with the principles of the Canadian Council on Animal Care (CCAC). The Animal Use Protocol (AUP 20090082) involved in the current study is reviewed annually, and approval is granted by the University of Saskatchewan’s Animal Research Ethics Board (AREB).
Figures




References
-
- Maser, R., Mitchell, B., Vinik, A. & Freeman, R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 26 (6), 1895–1901 (2003). - PubMed
-
- Vinik, A. I., Freeman, R. & Erbas, T. Diabetic autonomic neuropathy. Semin Neurol.23 (4), 365–372 (2003). - PubMed
-
- Tang, M., Donaghue, K. C., Cho, Y. H. & Craig, M. E. Autonomic neuropathy in young people with type 1 diabetes: A systematic review. Pediatr. Diabetes. 14 (4), 239–248 (2013). - PubMed
-
- Kuehl, M. & Stevens, M. J. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol. 8(7):405–16. http://www.ncbi.nlm.nih.gov/pubmed/22371159 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

