Adverse effects of dipyrone (Metamizole) use during pregnancy on offspring health: a systematic review and meta-analysis
- PMID: 40652183
- PMCID: PMC12255972
- DOI: 10.1186/s12884-025-07872-x
Adverse effects of dipyrone (Metamizole) use during pregnancy on offspring health: a systematic review and meta-analysis
Abstract
Background: This meta-analysis and systematic review aimed to review the health outcomes of offspring following dipyrone use during pregnancy.
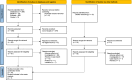
Methods: A systematic literature search was conducted in MEDLINE, Embase, and the Cochrane Library to identify clinical trials or observational studies investigating women who used dipyrone during pregnancy (up to 16 August 2024). The data was analyzed using odds ratios (ORs) with inverse variance and Bayesian random effects models.
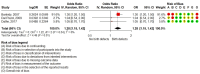
Results: Six case-control studies and four prospective cohort studies met the inclusion criteria. No association was found with birth defects (OR 1.27, 95% CI 0.83–1.77; I2 = 39.94%; 3 cohorts and 1 case-control), major anomalies (OR 1.06, 95% CI 0.47–2.37; I2 = 14.35%; 2 cohorts), infant leukemia (OR 1.25, 95% CI 0.86–2.22; I2 = 72.82%; 2 case-controls), gestational loss (OR 0.80, 95% CI 0.57–1.13; 3 cohorts), prematurity (OR 0.99, 95% CI 0.80–1.21; I2 = 0%; 3 cohorts), low birth weight, constriction of the ductus arteriosus, or renal and cardiac disorders. However, all analyses were of low or very low certainty.
Conclusion: No association was found between maternal use of dipyrone in the investigated outcomes, although these conclusions are limited by the quality of the included studies, sample size, and overall evidence. Further research with a better assessment of exposure is required to exclude risk.
Supplementary Information: The online version contains supplementary material available at 10.1186/s12884-025-07872-x.
Keywords: Congenital abnormalities; Dipyrone; Pregnancy.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Souza JP, Day LT, Rezende-Gomes AC, et al. A global analysis of the determinants of maternal health and transitions in maternal mortality. Lancet Glob Health. 2024;12(2):e306–16. 10.1016/S2214-109X(23)00468-0. - PubMed
-
- Zafeiri A, Mitchell RT, Hay DC, Fowler PA. Over-the-counter analgesics during pregnancy: a comprehensive review of global prevalence and offspring safety. Hum Reprod Update. 2021;27(1):67–95. 10.1093/humupd/dmaa042. - PubMed
-
- Werler MM, Mitchell AA, Hernandez-Diaz S, Honein MA. Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193(3 Pt 1):771–7. 10.1016/j.ajog.2005.02.100. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

