Guilty by association: direct interaction with the tetraspanin CD63 suggests a role for organic cation transporter 3 in histamine release from granulocytes
- PMID: 40652190
- PMCID: PMC12255121
- DOI: 10.1186/s12929-025-01158-2
Guilty by association: direct interaction with the tetraspanin CD63 suggests a role for organic cation transporter 3 in histamine release from granulocytes
Abstract
Background: The organic cation transporter 3 (OCT3) is a ubiquitous transporter that carries both endogenous and exogenous substrates, such as histamine and cisplatin. Our investigations have shown that OCT3 directly interacts with the tetraspanin CD63. CD63 is a marker for activated basophils and mast cells, which are granulocytes capable of rapidly releasing large amounts of histamine. This makes them key players in the development of allergic reactions.
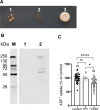
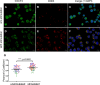
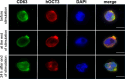
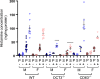
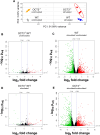
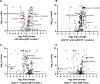
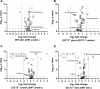
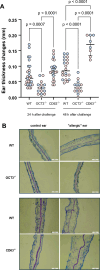
Methods and results: In this work, we demonstrated that OCT3 is present in murine and human basophils and is strongly colocalized with CD63 in a specific region of the plasma membrane, particularly after cell activation leading to histamine release. Furthermore, we confirmed that part of the histamine release from basophils is mediated by OCT3. In a mouse model of contact dermatitis, the presence of OCT3 is crucial for determining the severity of the allergic reaction. The presence of CD63 also seems to be important for regulating the allergic response, although it does not directly affect histamine secretion. RNA-Seq and metabolome analyses revealed that wild-type mice and mice with genetic deletion of OCT3 (OCT3-/-) are phenotypically very similar, and that the observed effects in OCT3-/- organisms can be attributed mainly to the genetic deletion of the OCT3 transporter.
Conclusions: In conclusion, OCT3 is a transporter for histamine in granulocytes, which plays a crucial role in determining the intensity of allergic reactions and may be a target for interventions aimed at reducing their severity.
Keywords: Allergy; CD63; Histamine; Organic cation transporter 3.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All experiments conducted in this study with animals were ethically approved by the North Rhine Westphalia State Environment Agency (LANUV, approval 81-02.04.2018.A387) and adhered strictly to national guidelines concerning animal welfare and protection. The ethics committee of the University of Münster approved all experiments performed with PBMC isolated from buffy coats (German Red Cross) (2013-286-f-S). We affirm that this manuscript has not been published elsewhere and is not under consideration by another journal. All authors have approved the manuscript and declare no conflicts of interest. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Busch AE, Karbach U, Miska D, Gorboulev V, Akhoundova A, Volk C, et al. Human neurons express the polyspecific cation transporter hOCT2, which translocates monoamine neurotransmitters, amantadine, and memantine. Mol Pharmacol. 1998;54:342–52. - PubMed
-
- Koepsell H. Update on drug-drug interaction at organic cation transporters: mechanisms, clinical impact, and proposal for advanced in vitro testing. Expert Opin Drug Metab Toxicol. 2021;17:635–53. - PubMed
-
- Thévenod F, Ciarimboli G, Leistner M, Wolff NA, Lee W-K, Schatz I, et al. Substrate- and cell contact-dependent inhibitor affinity of human organic cation transporter 2: studies with two classical organic cation substrates and the novel substrate Cd2+. Mol Pharm. 2013;10:3045–56. - PubMed
-
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–51. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

